Solutions
Solutions PDF Notes, Important Questions and Synopsis
SYNOPSIS
- Solutions are homogeneous mixtures of two or more components.
- Solute: The substance present in a smaller amount in a solution.
- Solvent: The substance present in a larger amount in a solution.
- Binary solutions: Solutions which contain two components.
- Types of Solutions:
Gaseous Solutions
Solute Solvent Common Examples Gas Gas Mixture of oxygen and nitrogen gases Liquid Gas Chloroform mixed with nitrogen gas Solid Gas Camphor in nitrogen gas
Liquid Solutions
Solute Solvent Common Examples Gas Liquid Oxygen dissolved in water Liquid Liquid Ethanol dissolved in water Solid Liquid Glucose dissolved in water
Solid Solutions
Solute Solvent Common Examples Gas Solid Solution of hydrogen in palladium Liquid Solid Amalgam of mercury with sodium Solid Solid Copper dissolved in gold -
Solubility: It is defined as the amount of solute dissolved in 100 g of solvent to form a saturated solution.
-
Solubility of a solid in a liquid:
Effect of temperature: The solubility of a solid in a liquid increases with increase in temperature if dissolution is endothermic.
If dissolution is exothermic, the solubility of a solid in liquid decreases with increase in temperature.
Effect of pressure: The solubility of a solid in a liquid is not affected significantly by pressure because solids and liquids cannot be compressed.b -
Saturated solution: A solution in which additional solute cannot be dissolved at the same temperature is called a saturated solution.
-
Unsaturated solution: A solution in which more amount of solute can be dissolved at the same temperature.
-
Supersaturated solution: A solution which contains more mass of the dissolved solute than the saturated solution at the same temperature and pressure.
-
Solubility of gases in a liquid:
Factors affecting the solubility of a gas in a liquid:- Nature of gas
- Nature of liquid
- Temperature
- Pressure
-
Henry’s Law:
The solubility of a gas in a liquid is directly proportional to the pressure of the gas. -
Vapour pressure of solutions of solids in liquids:
Raoult’s Law:
The partial vapour pressure of each volatile component in the solution is directly proportional to its mole fraction. -
Ideal solutions:
Solutions which obey Raoult’s law over the entire range of concentration.
Ideal solutions have two important properties:- Enthalpy of mixing of the pure components to form the solution is zero.
- Volume of mixing is zero.
-
Non-ideal solutions:
Solutions which do not obey Raoult’s law over the entire range of concentration. -
Azeotropic mixtures:
Some liquid solutions of two (or more) components have the same composition in solution as well as in the vapour phase. Such mixtures are azeotropic and known as azeotropes. -
Colligative properties:
These are properties of the solution which depend on the number of solute particles present in the solution irrespective of the nature of solute and solvent. -
Relative lowering of vapour pressure of solvent:
The vapour pressure of a solvent in a solution is less than that of a pure solvent. According to Raoult’s law, the relative lowering in the vapour pressure of dilute solution is equal to the mole fraction of solute (non-volatile) present in the solution. -
Elevation of boiling point:
The difference in boiling point of solution and pure solvent is called the elevation of boiling point. It is directly proportional to the number of moles of solute in a given amount of solvent. -
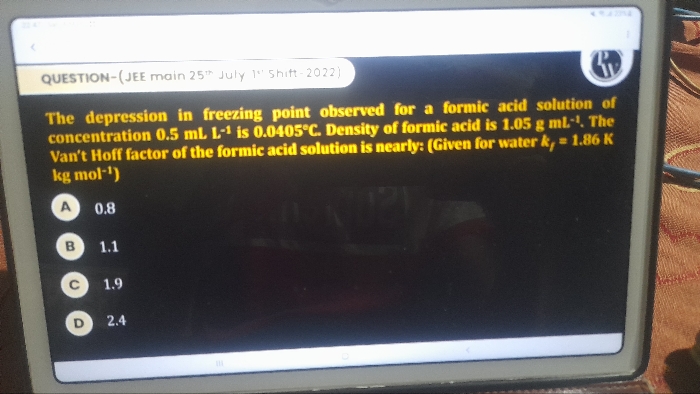
Depression in freezing point:
The difference between the freezing point of a pure solvent and solution is directly proportional to the number of moles of solute in a given amount of solvent. -
Osmosis:
Osmosis is the tendency of a solvent to move through a thin porous (semi-permeable) membrane from a dilute solution to a more concentrated solution. -
Isotonic solutions:
Two solutions are said to be isotonic when they exert the same osmotic pressure because they have the same molar concentration. -
Isosmotic solutions:
When two isotonic solutions are separated by a semi-permeable membrane, no osmosis occurs and the two isotonic solutions are called isosmotic solutions. -
Abnormal molecular mass:
When the molecular mass calculated with the help of a colligative property is different from the theoretical molecular mass, it is called abnormal molecular mass. -
Association:
When the number of solute particles is less than the expected number, the solute molecules associate and the process is known as association. -
Dissociation:
When the number of solute particles is more than the expected number, the solute particles dissociate and the process is known as dissociation. -
Degree of dissociation:
The ratio of the number of molecules dissociated to the total number of molecules. -
van’t Hoff Factor:
The ratio of the experimental value of the colligative property to the theoretical value.
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry