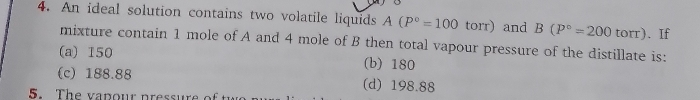JEE Class main Answered
Mole fraction of ch4 in a solution of 32g of ch4 and 256g of oxygen is?
Asked by atharvamane801 | 14 Jan, 2024, 12:07: PM
Dear Student,
Given mass of CH₄ = 32g
Molar mass of CH₄ = 16 g/mol
Moles of CH₄ = Mass / Molar mass
Moles of CH₄ = 32g / 16 g/mol ≈ 2 moles
Moles of O₂:
Given mass of O₂ = 256g
Molar mass of O₂ = 32 g/mol
Moles of O₂ = Mass / Molar mass
Moles of O₂ = 256g / 32 g/mol = 8 moles
Total moles in the solution = Moles of CH₄ + Moles of O₂
Total moles = 2 moles + 8 moles = 10 moles
The mole fraction of CH₄ = Moles of CH₄ / Total moles = 2 moles / 10 moles = 0.2
So, the mole fraction of CH₄ in the solution is 0.2.
Answered by | 16 Jan, 2024, 11:21: AM
JEE main - Chemistry
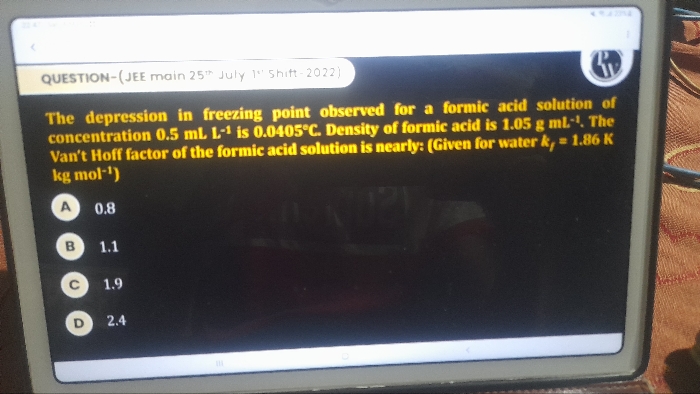
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM
JEE main - Chemistry
Asked by debnath.ankita2023 | 16 May, 2024, 13:35: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by atharvamane801 | 14 Jan, 2024, 12:07: PM
JEE main - Chemistry
Asked by bhyogita884 | 12 Jul, 2022, 02:55: AM
JEE main - Chemistry
Asked by abdulraqeeb437 | 16 Jun, 2022, 20:38: PM
JEE main - Chemistry
Asked by akshatmi2005 | 21 May, 2021, 14:23: PM