Co-ordination Compounds
Co-Ordination Compounds PDF Notes, Important Questions and Synopsis
SYNOPSIS
- Coordination compound: A coordination compound contains a central metal atom or ion surrounded by several oppositely charged ions or neutral molecules. These ions or molecules re-bonded to the metal atom or ion by a coordinate bond.
- Coordination entity: A coordination entity constitutes a central metal atom or ion bonded to a fixed number of ions or molecules. Example: In K4[Fe(CN)6], [Fe(CN)6]4− represents a coordination entity.
- Central atom or ion: In a coordination entity, the atom/ion to which a fixed number of ions/groups are bound in a definite geometrical arrangement is called the central atom or ion. Example: In K4[Fe(CN)6], Fe²+ is the central metal ion.
- Ligands: A molecule, ion or group which is bonded to the metal atom or ion in a complex or coordination compound by a coordinate bond is called a ligand. It may be neutral, positively or negatively charged. Examples: H2O, CN−, NO+ etc.
- Donor atom: An atom of the ligand attached directly to the metal is called the donor atom. Example: In the complex K4[Fe(CN)6], carbon is a donor atom.
- Coordination number: The coordination number (CN) of a metal ion in a complex can be defined as the number of ligand donor atoms to which the metal is directly bonded. Example: In the complex K4[Fe(CN)6], the coordination number of Fe is 6.
- Coordination sphere: The central atom/ion and the ligands attached to it are enclosed in square bracket and is collectively termed the coordination sphere. Example: In the complex K4[Fe(CN)6], [Fe(CN)6]4− is the coordination sphere.
- Counter ions: The ions present outside the coordination sphere are called counter ions. Example: In the complex K4[Fe(CN)6], K+ is the counter ion.
- Coordination polyhedron: The spatial arrangement of the ligand atoms which are directly attached to the central atom/ion defines a coordination polyhedron about the central atom. The most common coordination polyhedra are octahedral, square planar and tetrahedral. Examples: [PtCl4]2− is square planar, Ni(CO)4 is tetrahedral and [Cu(NH3)6]3+ is octahedral.
- Charge on the complex ion: The charge on the complex ion is equal to the algebraic sum of the charges on all the ligands coordinated to the central metal ion.
- Denticity: The number of ligating (linking) atoms present in a ligand is called denticity.
- Unidentate ligands: The ligands whose only donor atom is bonded to a metal atom are called unidentate ligands. Examples: H2O, NH3, CO, CN−
- Didentate ligands: The ligands which contain two donor atoms or ions through which they are bonded to the metal ion. Example: Ethylene diamine (H2NCH2CH2NH2) has two nitrogen atoms, and oxalate ion
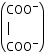 has two oxygen atoms which can bind with the metal atom.
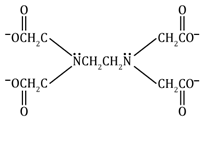
has two oxygen atoms which can bind with the metal atom. - Polydentate ligand: When several donor atoms are present in a single ligand, the ligand is called a polydentate ligand. Example: In N(CH2CH2NH2)3, the ligand is said to be polydentate. Ethylenediaminetetraacetate ion (EDTA4–) is an important hexadentate ligand. It can bind through two nitrogen and four oxygen atoms to a central metal ion.
- Chelate: An inorganic metal complex in which there is a close ring of atoms caused by attachment of a ligand to a metal atom at two points. An example is the complex ion formed between ethylene diamine and cupric ion [Cu(NH2CH2NH2)2]2+.
- Ambidentate ligands: Ligands which can ligate (link) through two different atoms present in it are called ambidentate ligands. Examples: NO2− and SCN−. NO2− can link through N as well as O, while SCN− can link through S as well as N.
Common Chelating Amines
|
Common Name |
IUPAC Name |
Abbreviation |
Formula |
|
Ethtlenediamine |
1,2-ethanediamine |
en |
NH2CH2CH2NH2 |
|
propanediamine |
1,2-propanediamine |
pn |
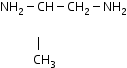 |
|
dithylenetriamine |
[N-(2-aminoethyl)-1 |
dien |
NH2CH2CH2NHCH2CH2NH3 |
|
triethylenetetraamine |
[N-(2-aminoethyl)-1 2-ethanediamine or triethylenetetraamine |
trien |
NH2CH2CH2NHCH2CH2NHCH2CH2NH2 |
|
triaminotriethylamine |
β,β’ ,β”-tris (2-aminoe-thyl)amine. |
tren |
NH2CH2CH2NHCH2CH2NHCH2CH2NH2 |
|
Teraethylene pentaamine |
1,4,710 pentaazatridecane or tetraethylenepentaamine |
|
NH2CH2CH2NHCH2CH2NHCH2CH2NHCH2 |
|
Ethylenediamine tetraacetate |
1,2-ethandiyl (dinitro tetraacetate or ethylenediaminetetraacetate |
EDTA |
|
|
Common Name |
Abbreviation |
Formula |
Structure |
|
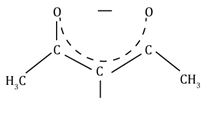
Acetyl acetonato |
acac |
CH3COCHCOCH3⎺
|
 |
|
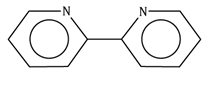
2,2'-bipyridine |
bipy |
C10H8N2 |
|
|
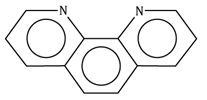
1,10-phenanthroline/ phenanthroline |
Phen’o-phen |
C12H8N2 |
|
|
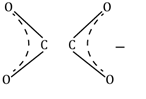
oxalato |
ox |
C2O42⎺ |
 |
|
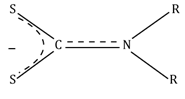
Dialkyl dithiocarbamato |
dtc |
S2CNR2⎺ |
 |
|
1,2-bis(diphenyl phophine)ethane |
dppe |
Ph2PC2H4PPh2 |
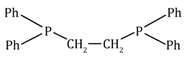 |
|
o-phenylenebis (dimethylarsine) |
diars |
C6H4(As(CH3)2)2 |
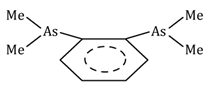 |
|
Dimethyl glyoximato |
DMG |
HONC(CH3)C(CH3)NO⎺ |
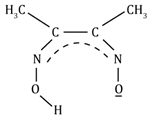 |
|
Ethylenediamine tetraacetato |
EDTA |
(⎺OCCH2)2NCH2CH2N(CH2COO⎺)2
|
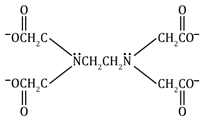 |
|
pyrazolylborato |
|
|
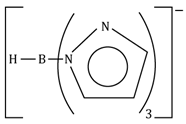 |
- Werner’s coordination theory:Werner was able to explain the nature of bonding in complexes. The postulates of Werner’s theory are
- Metal shows two kinds of valencies—primary valence and secondary valence.
- The ions/groups bound by secondary linkages to the metal have characteristic spatial arrangements corresponding to different coordination numbers.
- The most common geometrical shapes in coordination compounds are octahedral, square planar and tetrahedral.
- Oxidation number of the central atom: The oxidation number of the central atom in a complex is defined as the charge it would carry if all the ligands are removed along with the electron pairs which are shared with the central atom.
- Homoleptic complexes: Those complexes in which metal or ion is coordinately bonded to only one kind of donor atom. Example: [Co(NH3)6]3+
- Heteroleptic complexes: Those complexes in which metal or ion is coordinately bonded to more than one kind of donor atom. Example: [CoCl2(NH3)4]+, [Co(NH3)5Br]2+
- Isomers: Two or more compounds which have the same chemical formula but different arrangement of atoms are called isomers.
- Types of isomerism
- Structural isomerism
- Linkage isomerism
- Solvate isomerism or hydrate isomerism
- Ionisation isomerism
- Coordination isomerism
- Stereoisomerism
- Geometrical isomerism
- Optical isomerism
- Structural isomerism: This type of isomerism arises due to the difference in structures of coordination compounds. Structural isomerism, or constitutional isomerism, is a form of isomerism in which molecules with the same molecular formula have atoms bonded together in different orders.
- Ionisation isomerism: This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become the counter ion.
- Examples: [Co(NH3)5Br] SO4 and [Co(NH3)5 SO4] Br
- Solvate isomerism: It is isomerism in which the solvent is involved as the ligand. If the solvent is water, then it is called hydrate isomerism.
- Example: [Cr(H2O)6]Cl3 and [CrCl2(H2O)4] Cl2.2H2O
- Linkage isomerism: Linkage isomerism arises in a coordination compound containing an ambidentate ligand. In the isomerism, a ligand can form linkage with metal through different atoms.
- Examples: [Co(NH3)5ONO]Cl2 and [Co(NH3)5NO2]Cl2
- Coordination isomerism: This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex. Examples: [Co(NH3)6][Cr(C2O4)3] and [Cr(NH3)6][Co(C2O4)3]
- Stereoisomerism:This type of isomerism arises because of different spatial arrangement.
- Geometrical isomerism: It arises in heteroleptic complexes due to different possible geometrical arrangements of ligands.
- Optical isomerism:Optical isomers are those isomers which are non-superimposable mirror images.
- Valence bond theory:
According to this theory, the metal atom or ion under the influence of ligands can use its (n − 1)d, ns, np or ns, np or nd orbitals for hybridisation to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral and square planar.
These hybridised orbitals are allowed to overlap with ligand orbitals which can donate electron pairs for bonding.
|
Coordination number |
Type of hybridisation |
Distribution of hybrid orbitals in space |
|---|---|---|
|
4 |
sp3 |
Tetrahedral |
|
4 |
dsp2 |
Square planar |
|
5 |
sp3d |
Trigonal bipyramidal |
|
6 |
sp3d2 (nd orbitals are involved; outer orbital complex or high-spin or spin-free complex) |
Octahedral |
|
6 |
d2sp3 [(n − 1)d orbitals are involved; inner orbital complex or low-spin or spin-paired complex] |
Octahedral |
- Magnetic properties of coordination compounds: A coordination compound is paramagnetic in nature if it has unpaired electrons and diamagnetic if all the electrons in the coordination compound are paired.
Magnetic moment ,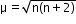 where n is the number of unpaired electrons.
where n is the number of unpaired electrons. - Crystal Field Theory: It assumes the ligands to be point charges and there is an electrostatic force of attraction between ligands and the metal atom or ion. It is a theoretical assumption.
- Crystal field splitting in octahedral coordination complexes
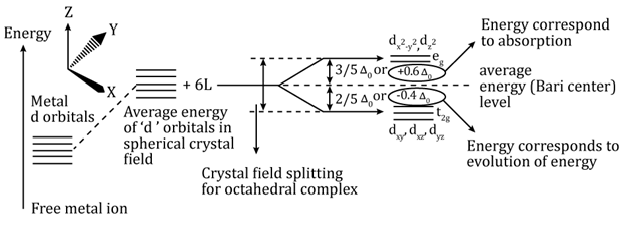
- Crystal field splitting in tetrahedral coordination complexes
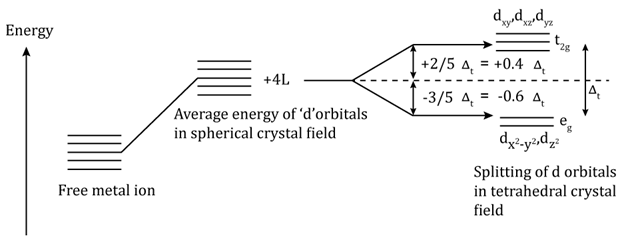
- For the same metal, the same ligands and metal–ligand distances, the difference in energy between eg and t2g level is
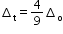
- Metal carbonyls: Metal carbonyls are homoleptic complexes in which carbon monoxide (CO) acts as the ligand. Example: Ni(CO)4
The metal–carbon bond in metal carbonyls possesses both characters. The metal–carbon bond in metal carbonyls possess both s and p characters. The M–C σ bond is formed by the donation of a lone pair of electrons from the carbonyl carbon into a vacant orbital of the metal. The M–C Π bond is formed by the donation of a pair of electrons from a filled d orbital of metal into the vacant anti-bonding Π* orbital of carbon monoxide. The metal to ligand bonding creates a synergic effect which strengthens the bond between CO and the metal.
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry