s-Block Element (Alkali and Alkaline Earth Metals)
s Block Element PDF Notes, Important Questions and Synopsis
SYNOPSIS
Hydration enthalpy:
Hydration enthalpy of alkali metal ions decreases with increase in ionic size.
Physical properties:
- All alkali metals are silvery white, soft and light metals. Because of their large size, these elements have low density.
- Melting and boiling points of alkali metals are low, indicating weak metallic bonding.
- Alkali metals and their salts impart a characteristic colour to the oxidising flame.
Metal
Li
Na
K
Rb
Cs
Colour
Crimson red
Yellow
Pale violet
Red violet
Blue
Chemical properties:
Alkali metals are highly reactive due to their large size and low ionisation enthalpy.
- Reactivity towards air: They burn vigorously in oxygen forming oxides. Lithium forms monoxide, sodium forms peroxide, and the other metals form superoxide.
- Reducing nature: They are strong reducing agents; lithium showing the most reducing nature and sodium showing the least reducing nature.
-
Solution in liquid ammonia: They dissolve in liquid ammonia to produce a deep blue solution which is conducting in nature.
The blue colour of the solution is due to the ammoniated electron, and the solution is paramagnetic.
In concentrated solution, the blue colour changes to bronze colour and becomes diamagnetic.
ANOMALOUS PROPERTIES OF LITHIUM
Although the lithium atom and ion are exceptionally small, it has high polarising power (i.e. charge–radius ratio).
GROUP 2 ELEMENTS: ALKALINE EARTH METALS
Hydration enthalpies:
- Order of hydration enthalpies of alkaline earth metal ions: Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+
- The hydration enthalpies of alkaline earth metal ions are higher than those of alkali metal ions. Thus, compounds of alkaline earth metals are more extensively hydrated compared to alkali metals, e.g. MgCl2 and CaCl2 exist as MgCl2.6H2O and CaCl2.6H2O, while NaCl and KCl do not form such hydrates.
- Alkaline earth metals, in general, are silvery white, lustrous and relatively soft but harder than alkali metals.
- Melting and boiling points of Be and Mg are high due to their small size. Hence, these elements do not impart any colour to the flame.
Metal
Be
Mg
Ca
Sr
Ba
Colour
No
Colour
No Colour
Brick red
Crimson
Apple green
- Calcium, strontium and barium impart characteristic colour to the flame.
- Reactivity towards air and water: Beryllium and magnesium are inert to oxygen and water. Magnesium is more electropositive and burns with dazzling brilliance in air to give MgO and Mg3N2. Calcium, strontium and barium are readily oxidised in air to form their oxides and nitride.
- Reducing nature: Alkaline earth metals are strong reducing agents. This is indicated by the large negative value of their reduction potentials.
- Solution in liquid ammonia: Alkaline earth metals dissolve in liquid ammonia to give a deep blue black solution forming ammoniated ions.
M + (x + y) NH3 → [M(NH3)x]2++ 2[e(NH3)Y]
Ammoniates [M(NH3)6]2+ can be recovered from these solutions.
ANOMALOUS BEHAVIOUR OF BERYLLIUM
Beryllium, the first member of the Group 2 metals, shows anomalous behaviour as compared to magnesium and the rest of the members. It shows a diagonal relationship with aluminium.
Diagonal Relationship between Beryllium and Aluminium
The ionic radius of Be2+ is estimated to be 31 pm; the charge–radius ratio is nearly the same as that of the Al3+ ion. Hence, beryllium resembles aluminium in some ways.
- Compounds of s-block elements:
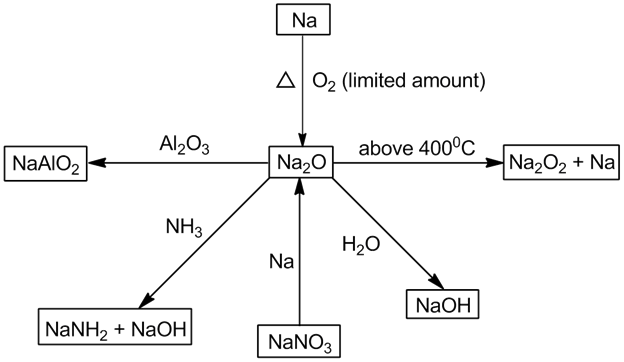
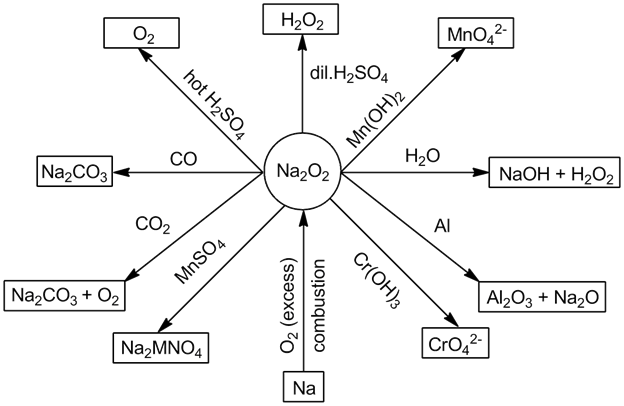
- Sodium peroxide (Na2O2):
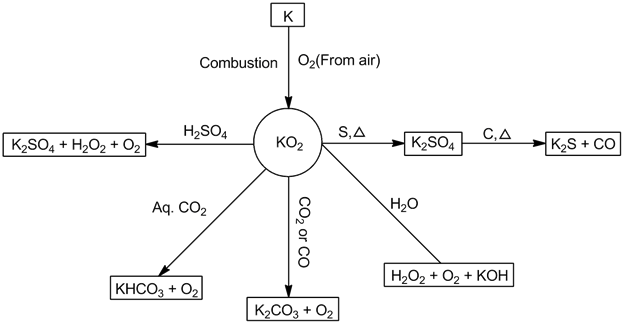
- Potassium superoxide (KO2):
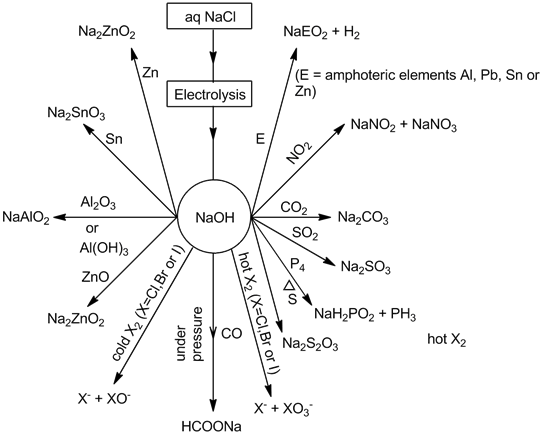
- Sodium hydroxide (NaOH):
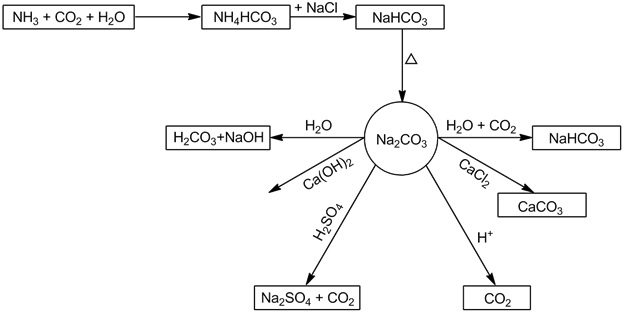
- Sodium carbonate (Na2CO3):
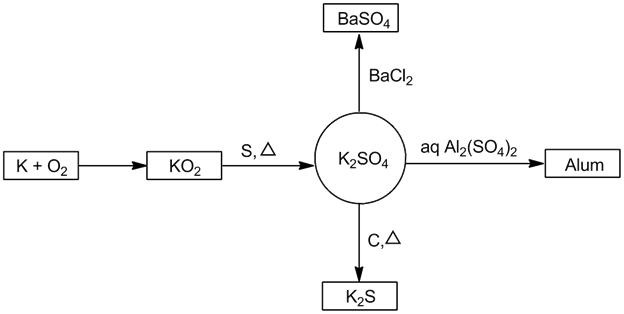
- Potassium sulphate (K2SO4):
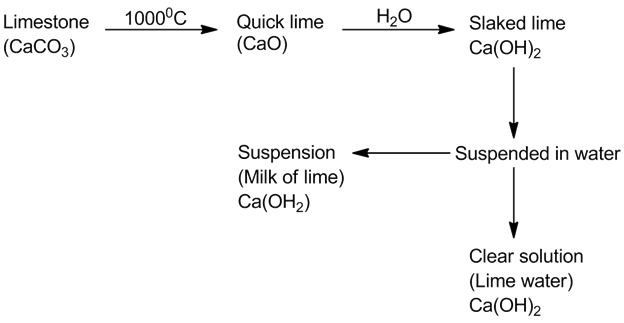
- Quick lime, slaked lime and lime water:
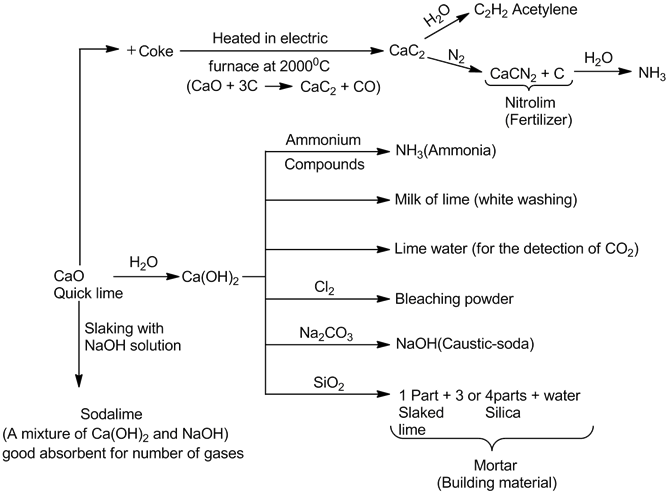
3Ca(OH)2 + 2Cl2 → Ca(OCl)2. Ca(OH)2. CaCl2. 2H2O (bleaching powder)
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry

