Surface Chemistry
Surface Chemistry PDF Notes, Important Questions and Synopsis
SYNOPSIS
- Adsorption: The phenomenon of attracting and retaining the molecules of a substance on the surface of a liquid or solid resulting in a higher concentration of the molecules on the surface.
- Adsorbate: Substance adsorbed on the surface.
- Adsorbent: Substance on which the adsorbate is adsorbed.
- Desorption: Removal of the adsorbed substance from the surface.
- Occlusion: Adsorption of gases on the surface of metals.
- Entropy change during adsorption:
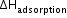 is always negative. During adsorption, molecules are adsorbed on the surface of a solid adsorbent; hence, entropy decreases, i.e. ΔS is also negative. As ΔG = ΔH - TΔS, the process of adsorption to occur, ΔG must be negative.
is always negative. During adsorption, molecules are adsorbed on the surface of a solid adsorbent; hence, entropy decreases, i.e. ΔS is also negative. As ΔG = ΔH - TΔS, the process of adsorption to occur, ΔG must be negative. - Adsorption equilibrium:
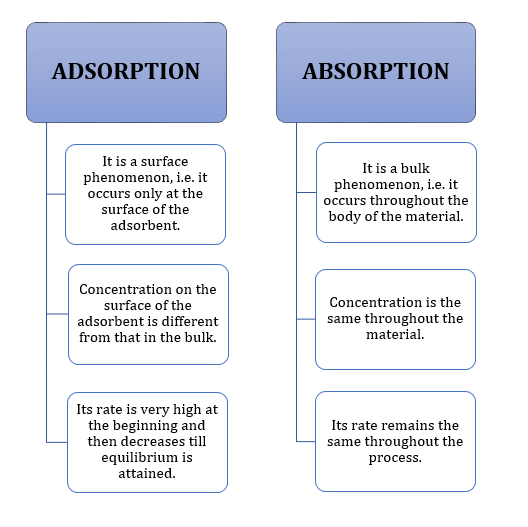
- Difference between adsorption and absorption:
-
Positive Adsorption:
Concentration of the adsorbate is more on the surface of the adsorbent than in the bulk. -
Negative Adsorption:
Concentration of the adsorbate increases in the bulk after adsorption.
-
Physical Adsorption Vs Chemical Adsorption:
Physical Adsorption
Chemical Adsorption
Weak Van der Waals forces are present.
Strong chemical interactions are present.
Energy of adsorption is low.
Energy of adsorption is high.
Process takes place at low temperature.
Process takes place at high temperature.
It is not specific.
It is highly specific.
Process is reversible.
Process is irreversible.
Occurs rapidly.
Occurs slowly.
-
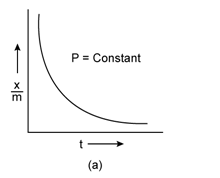
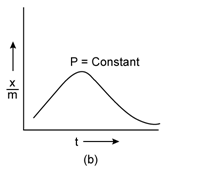
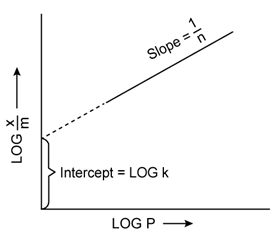
Freundlich Adsorption Isotherm:
A graph between the amount of the gas adsorbed per gram of the adsorbent (x/m) and the equilibrium pressure of the adsorbate at constant temperature is called the adsorption isotherm.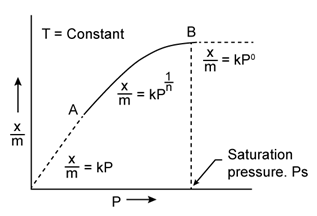
-
Catalysis: A catalyst is a substance which can alter the speed of a chemical reaction without itself undergoing any change in mass and its composition. This phenomenon is known as catalysis.
-
Positive catalyst: If the catalyst increases the speed of reaction, then it is called a positive catalyst, and the phenomenon is called positive catalysis.
-
Negative catalyst: If a catalyst decreases the rate of reaction, then it is called a negative catalyst, and the phenomenon is called negative catalysis.Types of catalysis:
Homogeneous catalysis: If the catalyst is present in the same phase as the reactants, it is called homogeneous catalyst, and this type of catalysis is called homogeneous catalysis.
Heterogeneous catalysis: If the catalyst is present in the different phase from that of the reactants, it is called a heterogeneous catalyst, and this type of catalysis is called heterogeneous catalysis.
- Modern Adsorption Theory:
- Diffusion of reactant molecules towards the surface of the catalyst.
- Adsorption of the reactant molecules on the surface of the catalyst by forming a loose bond with the catalyst due to the presence of free valencies.
- Occurrence of the chemical reaction between the reactants and the catalyst forming an intermediate.
- Desorption of the product molecule from the surface due to lack of its affinity for the catalyst surface, thereby making the surface free for fresh adsorption of reactant molecules.
- Diffusion of product molecules away from the surface of the catalyst.
- Important features of a solid catalyst:
- Activity: Capacity to increase the speed of a chemical reaction.
- Selectivity: Selectivity of a catalyst is the ability of a catalyst to direct the reaction to form a particular product excluding others.
- Specificity: The given substance can act as a catalyst only for specific reactions, not for all reactions
-
Shape-selective catalysis by zeolites:
The catalytic reaction which depends on the pore structure of the catalyst and the size of reactant and product molecules is called shape-selective catalysis. -
Enzyme catalysis:
Enzymes are defined as biochemical catalysts and globular proteins with high molar mass.
Characteristics of enzymes:- Specificity
- Efficiency
- Small quantity
- Highly active under optimum temperature and pH
- Increasing activity in the presence of activators and co-enzymes
- Influence of inhibitors and poisons
Mechanism: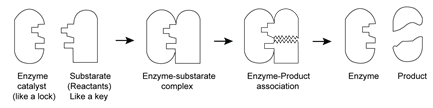
-
Colloids:
A colloid is a homogeneous system in which one substance is dispersed as very fine particles in another substance called a dispersion medium. -
Classification of colloids based on the physical state of the dispersed phase and the dispersed medium:
Dispersed phase Dispersion medium Type of colloid Examples Solid Solid Solid sol Some coloured glasses, gem stones Solid Liquid Sol Paints, cell fluids
Solid Gas Aerosol Smoke, dust Liquid Solid Gel Cheese, butter, jellies Liquid Liquid Emulsion Milk, hair cream Liquid Gas Aerosol Fog, mist, cloud Gas Solid Solid sol Pumice stone, foam rubber Gas Liquid Foam Froth, whipped cream, soap lather
- Classification of colloids based on the nature of interaction between the dispersed phase and the dispersion medium:
- Lyophilic: Liquid-loving
- Lyophobic: Liquid-hating
- Classification of colloids based on the type of particles of the dispersed phase:
- Multimolecular colloids
- Macromolecular colloids
- Associated colloids (micelles)
- Micelles:
There are some substances which at low concentrations behave as normal strong electrolytes, but at higher concentrations exhibit colloidal behaviour due to the formation of aggregates. The aggregate particles are called micelles.
This aggregate solution is formed during the cleaning action of soap.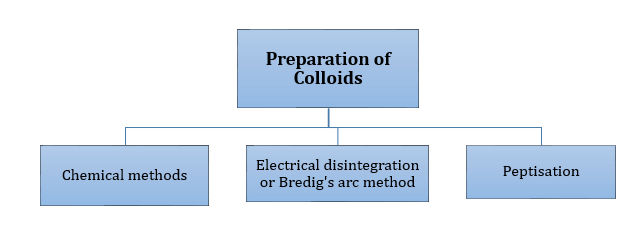
-
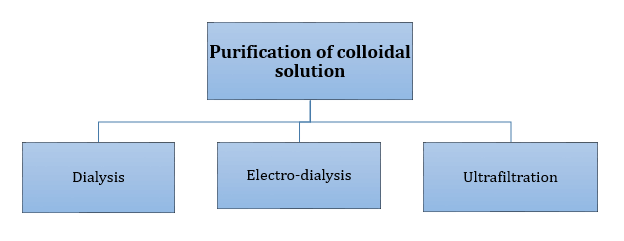
Properties of Colloidal Solutions:
- Colligative properties
- Colour
- Tyndall effect
- Brownian movement
- Charge on colloidal particles
- Electrophoresis
- Hardy–Schulze Law:
Hardy–Schulze Law: The quantity of the electrolyte which is required to coagulate a definite amount of a colloidal solution depends on the valency of the coagulating ion. Greater the valency of the coagulating or flocculating ion, greater is its power to bring about coagulation. -
Emulsions:
These are liquid–liquid colloidal systems.
There are two types of emulsions:
- Oil dispersed in water (O/W type)
- Water dispersed in oil (W/O type)
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry

