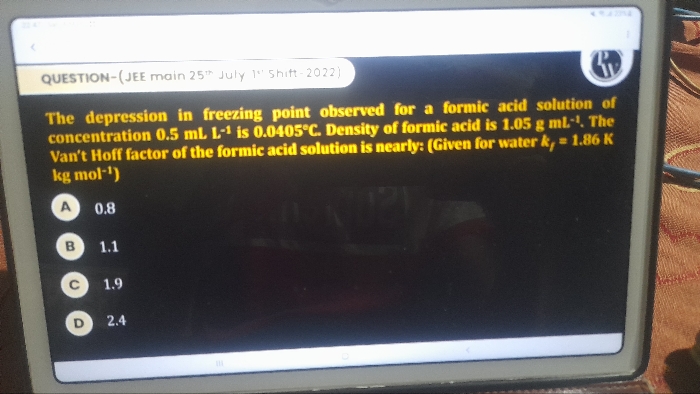
JEE Class main Answered
Sir while solving ,plz tell me each term of formula being applied here and the calculations .

Asked by vishakhachandan026 | 16 Aug, 2019, 19:47: PM
Correct option is (2)
Given:
Initial vapour density of PCl5 = 104.25
Vapor density at equilibrium = 60
Equilibrium equation is given as,
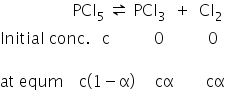
Total no. of moles = c(1−α) + cα + cα
= c(1+α)
We know,
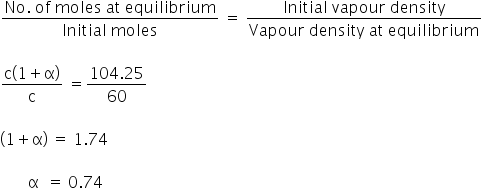
Degree of dissociation is 0.74
Answered by Varsha | 19 Aug, 2019, 16:52: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM
JEE main - Chemistry
Asked by debnath.ankita2023 | 16 May, 2024, 13:35: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by atharvamane801 | 14 Jan, 2024, 12:07: PM
JEE main - Chemistry
Asked by bhyogita884 | 12 Jul, 2022, 02:55: AM
JEE main - Chemistry
Asked by abdulraqeeb437 | 16 Jun, 2022, 20:38: PM
JEE main - Chemistry
Asked by akshatmi2005 | 21 May, 2021, 14:23: PM