Some Basic Principles of Organic Chemistry
Some Basic Principles of Organic Chemistry PDF Notes, Important Questions and Synopsis
SYNOPSIS
- All organic compounds are made up of carbon and hydrogen essentially.
Type of bond
Hybridisation
Bond angle
Structure
C-C single bond
sp3
109°28’
Tetrahedral
C=C double bond
sp2
120°
Triagonal planar
C≡C triple bond
sp
180°
Linear
- The order of s-character of these hybridized orbital is sp3 > sp2 > sp.
Characteristic of these hybridized orbital
Increasing order
s-character
sp3 > sp2 > sp
Bond length
sp3 > sp2 > sp
Bond energy or bond strength
sp> sp2 > sp3
- IUPAC rules for nomenclature of organic compounds:
- Functional group: An atom or a group of atoms present in the molecules, which determines the characteristics property of the organic compounds, is called the functional group.
Organic compound
Functional group
General formulae
Secondary suffix/Prefix
Haloalkanes
Halide -X
(F,Cl,Br,I)
R-X
Halo
Alcohols
Hydroxyl -OH
R-OH
-ol
Aldehydes
Aldehyde -CHO
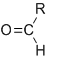
-al
Carboxylic acids
Carboxyl -COOH
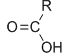
-oic acid
Ketones
Keto
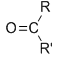
-one
Ethers
Ethers
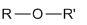
R-O-R’
-
Isomerism: The phenomenon of existence of two or more compounds possessing the same molecular formula but different
properties is known as isomerism. Such compounds are called as isomers.
Different types of isomerism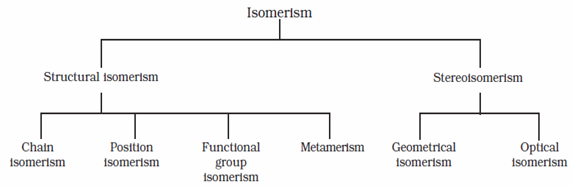
- Structural isomerism: Compounds having same molecular formula but different structures, i.e. arrangement of atoms or groups of atoms within molecules are called structural isomers and the phenomenon is called structural isomerism.
- Chain isomerism: Compounds having the same molecular formula but a different arrangement of carbon chains (skeletons) within the molecule are called chain isomers, and the phenomenon is termed as chain isomerism.
Example: Chain isomers of
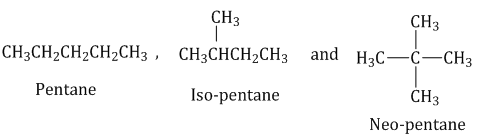
-
Position isomerism: Compounds which have the same molecular formula but differ in the position of the functional groups, carbon–carbon multiple bonds or substituent groups are called position isomers and the phenomenon is termed as position isomerism.
Example: Position isomers of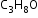 :
: 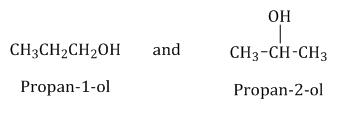
-
Functional groups isomerism: Compounds having the same molecular formula but different functional groups in the molecule are called functional group isomers, and this phenomenon is termed as functional group isomerism.
Example: Functional groups isomers of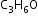
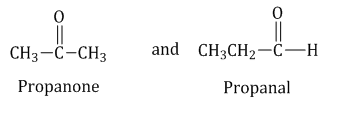
-
Metamerism: Compounds having same molecular formula but different number of carbon atoms (or alkyl groups) on either side of the functional group are called metamers, and this phenomenon is called metamerism.
Example: Metamers of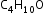
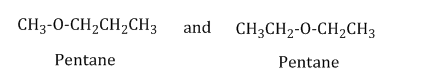
- Stereo isomerism: The isomers which have the same structural formula but have different relative arrangement of atoms or groups of atoms in space are called stereo isomers, and the phenomenon is called stereo isomerism.
Classification of stereoisomerism
- Geometrical isomerism: Isomerism due to the difference in spatial arrangements of groups about the doubly bonded carbon atoms is known as geometrical isomerism.
Example: Geometrical isomers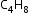
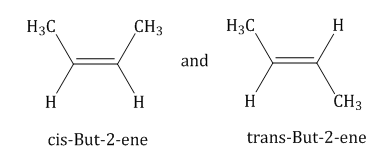
- Optical isomerism: Stereo isomers involving the arrangement of substituents about an asymmetric carbon atom or atoms so that the various isomers differ in the way they rotate a plane of polarized light are called optical isomers, and the phenomenon is called optical isomerism.
- Tautomerism: It is a special type of functional isomerism in which the isomers differ in the arrangement of atoms but they exist in dynamic equilibrium with each other, and this phenomenon is termed as tautomerism. Example: Acetaldehyde and vinyl alcohol are tautomers and exist in equilibrium as shown.
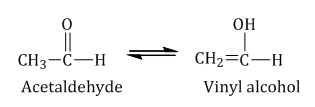
- Reaction mechanism: Reaction mechanism is a sequential account of each step describing the details of electron movement, energetics during bond cleavage and bond formation and the rates of transformation of reactants into products (kinetics).
- Reaction intermediates: Species produced during cleavage of bonds
Name of the species
Order of stability of the species
Reason for stability order
Free radical: An atom or group of atoms having an unpaired electron.
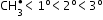
Larger the number of alkyl groups attached to the carbon atom carrying the odd electron, greater is the delocalization of the odd electron and hence more stable is the free radical.
Carbocation: Group of atoms which contain positively charged carbon having only six electrons (sextet of electrons)
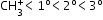
More the number of alkyl groups, the greater will be the dispersal of charge, and therefore carbocation will be more stable.
Carbanion: Group of atoms which contain negatively charged carbon carrying negative charge.
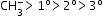
Larger the number of alkyl groups attached to the negatively charged carbon atom, greater will be the electron density on the carbon atom and lower will be its stability.
-
Different types of attacking reagent
Attacking Reagent
Examples
Free radical: An atom or group of atoms having an unpaired electron.
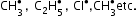
Electrophile: Positively charged or neutral species which are electron deficient and can accept a pair of electrons, i.e. an electrophile takes away an electron pair.
Positively charged:
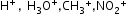 etc.
etc.Neutral:
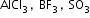 etc.
etc.Nucleophile: Nucleophile is a species that is electron rich and looks for electron deficient sites, i.e. nucleus loving or nucleus seeking (Nu), i.e. a nucleophile brings an electron pair with it.
Negatively charged:
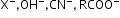 etc.
etc.Neutral:
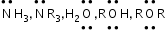
-
Electron displacement in covalent compounds
- Inductive effect: The process of electron displacement along the chain of carbon atoms due to the presence of a polar covalent bond at one end of the chain is called the inductive effect (I-effect); it is a permanent effect.
Note: I-effect decreases on moving away from the atoms involved in the initial polar bond and becomes negligible from the fourth atom onwards.
For comparing the relative effects, hydrogen is taken as a standard and the atoms or groups can be classified into two categories:
- Atoms or groups of atoms having electron-attracting more than hydrogen are referred to as having −I-effect (electron withdrawing or attracting).
Example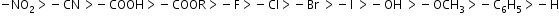
-
Atoms or groups of atoms having smaller electron attracting power than hydrogen are referred to as having +I-effect (electron donating or repelling).
Example: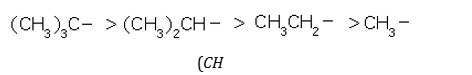
- Atoms or groups of atoms having electron-attracting more than hydrogen are referred to as having −I-effect (electron withdrawing or attracting).
-
Electromeric effect: It is a temporary effect which takes place between two atoms joined by a multiple bond, i.e. a double or triple bond. This occurs at the requirements of the attacking reagent and involves instantaneous transfer of a shared pair of electrons of the multiple bonds to one of the linked atoms.
The electromeric effect is classified as +E effect and −E effect:-
When the
 electrons of multiple bonds are transferred to that atom to which the reagent gets attached, it is called +E effect (positive electromeric).
electrons of multiple bonds are transferred to that atom to which the reagent gets attached, it is called +E effect (positive electromeric).
Example: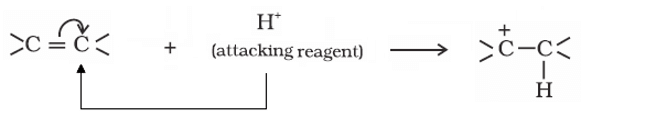
-
When the
 electrons of the multiple bonds are transferred to that atom to which the attacking reagent does not get attached, it is called −E (negative electromeric) effect.
electrons of the multiple bonds are transferred to that atom to which the attacking reagent does not get attached, it is called −E (negative electromeric) effect.
Example:
-
-
Resonance or mesomeric effect: If a molecule is assigned two or more Lewis structures, none of which is capable of describing all the known properties of the compound, then the actual structure is intermediate or resonance hybrid of these structures. This phenomenon is called resonance. The various structures written are called resonating structures.
Example: Resonating structures of CO2 are shown below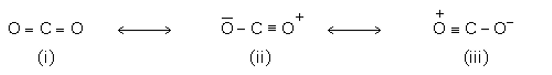
Resonance effect is classified as +R effect and −R effect-
If a substituent has tendency to donate electrons to double bond or conjugated system, then the effect is called the positive resonance effect or +R effect. Groups such as
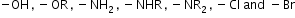 show +R effect.
show +R effect. -
If a substituent has tendency to withdraw electrons from a double bond or conjugated system towards itself, then the effect is called the negative resonance effect or −R effect. Groups such as
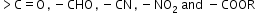 show −R effect.
show −R effect.
-
-
Hyperconjugation: It involves delocalisation of (s) electrons of C–H bond of an alkyl group directly attached to an atom of an unsaturated system or to an atom with an unshared p-orbital. The (s) electrons of C–H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unshared p-orbital. The interaction between the electrons of p systems (multiple bonds) and adjacent bonds (single H–C bonds) of the substituent groups in organic compounds is called hypercojugation. It is a permanent effect.
Example: Hypercojugation in propene
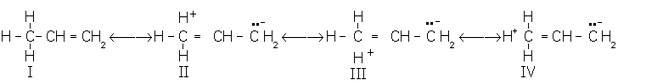
Because there is no bond between the -carbon atom and one of the hydrogen atoms, hyperconjugation is also called no-bond resonance. Although a free proton has been shown in the above structures, it is still bound quite firmly to the -cloud, and hence it is not free to move.
Order of hyperconjugation: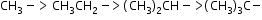
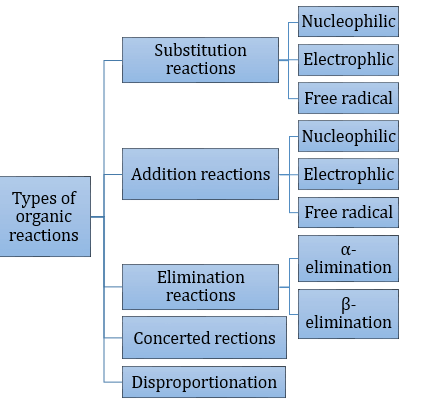
- Types of organic reactions:
Related Chapters
- Some Basic Concepts in Chemistry
- States of Matter
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry