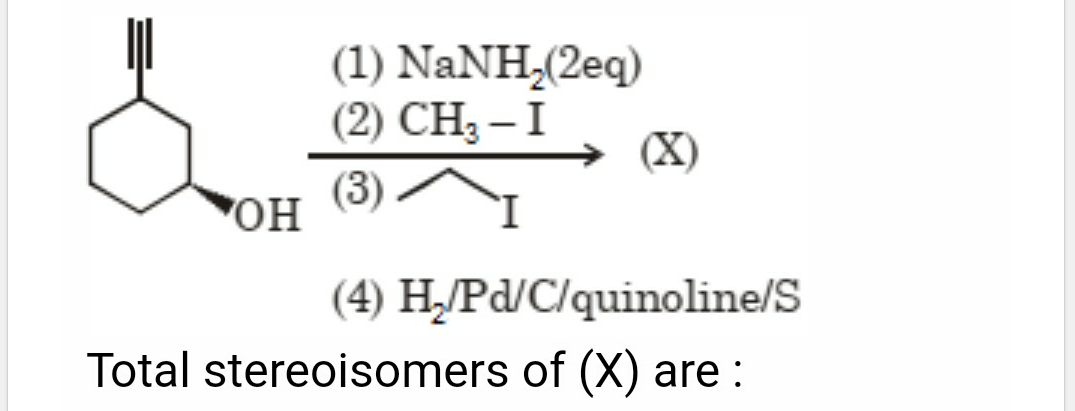JEE Class main Answered
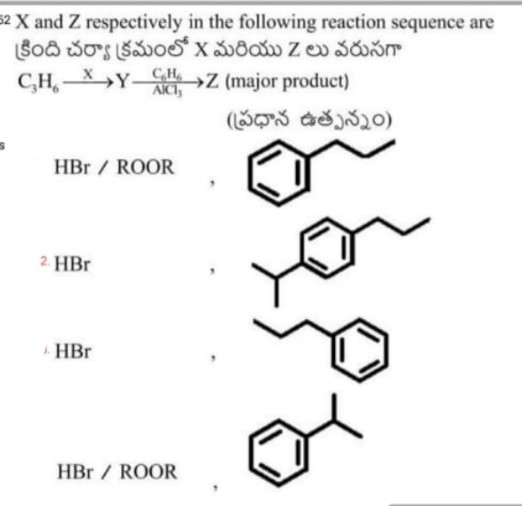
Q43
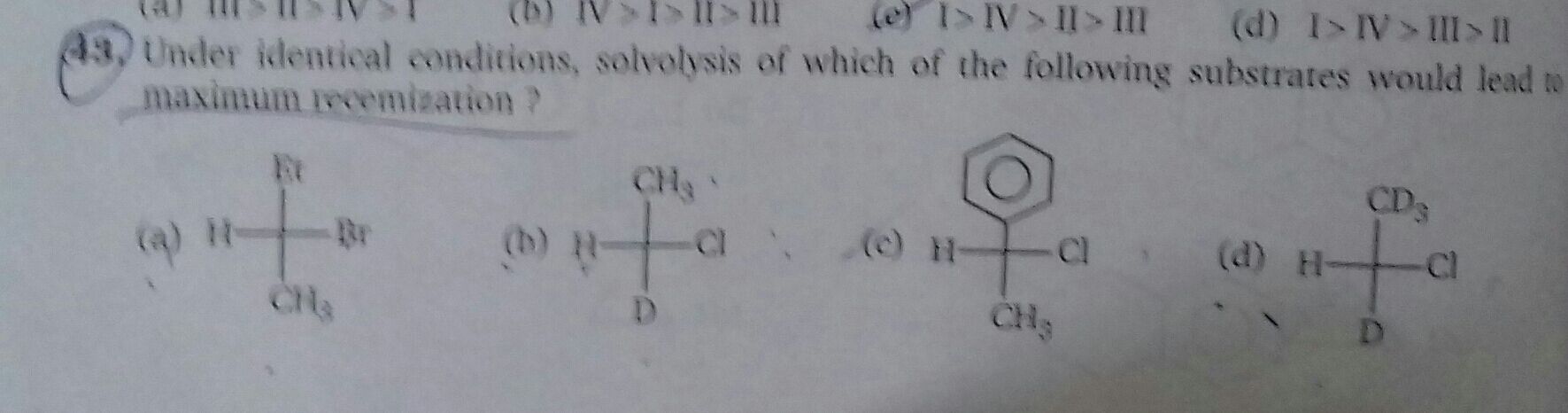
Asked by mdfaraz1182 | 11 Apr, 2020, 19:49: PM
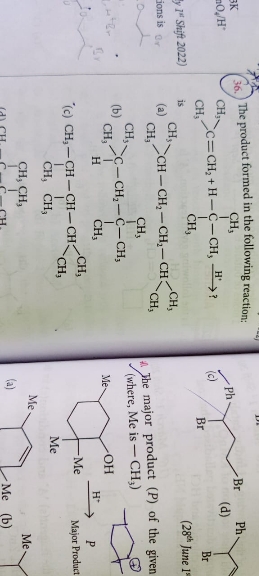
Solvolysis=SN1 reaction =intermediate=formation of carbocation=most stable carbocation = leads to maximum racemisation.
From the above chain of action it is clear that the most stable carbocation will gives us maximum racemisation.
Benzylic carbocation>allylic carbocation>tertiary carbocation> seconsary carbocation>primary >methyl
option 1 = This compound has electron wihdrawing Br group and formed secondary carbocation hence this option is omitted
option 2: Same as above Cl is EWG and formed primary carboccation hence omitted
option 4:Same as above
option 3: This compound has a phenyl ring attached hence the benzylic carbocation can be stabilised through resonance.
Hence compound in option 3 on undergoing solvolysis give maximum racemisation.
Answered by Ramandeep | 13 Apr, 2020, 12:36: PM
JEE main - Chemistry
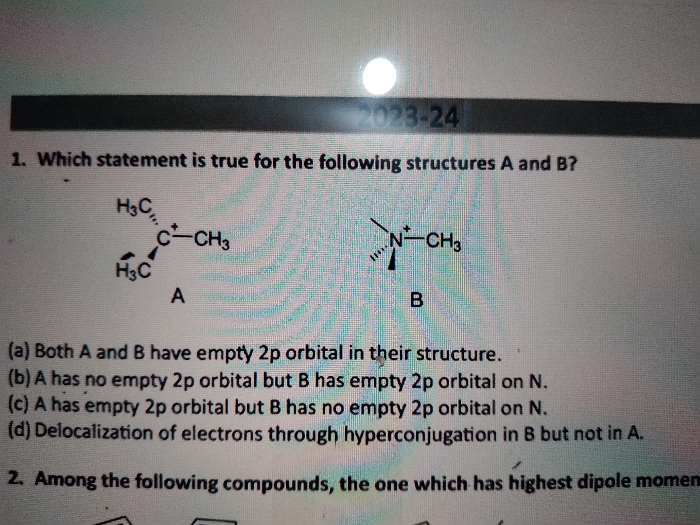
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by amanniju2 | 15 May, 2024, 09:05: AM
JEE main - Chemistry
Asked by srkgb8018 | 10 May, 2024, 07:36: AM
JEE main - Chemistry
Asked by jacksparrow7352133590 | 09 May, 2024, 20:20: PM
JEE main - Chemistry
Asked by patelamrutbhaib24 | 28 Jan, 2024, 13:44: PM
JEE main - Chemistry
Asked by harshakancharla329 | 27 Jan, 2024, 09:07: AM
JEE main - Chemistry
Asked by mp797056 | 23 Oct, 2023, 16:23: PM
JEE main - Chemistry
Asked by dheerajrao2005 | 26 Mar, 2022, 19:06: PM
JEE main - Chemistry
Asked by yasharthshankar | 05 Jun, 2020, 23:39: PM