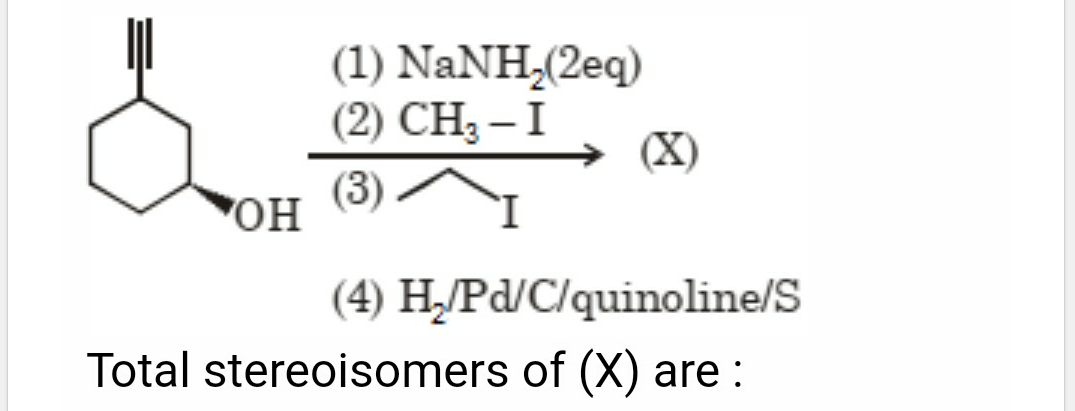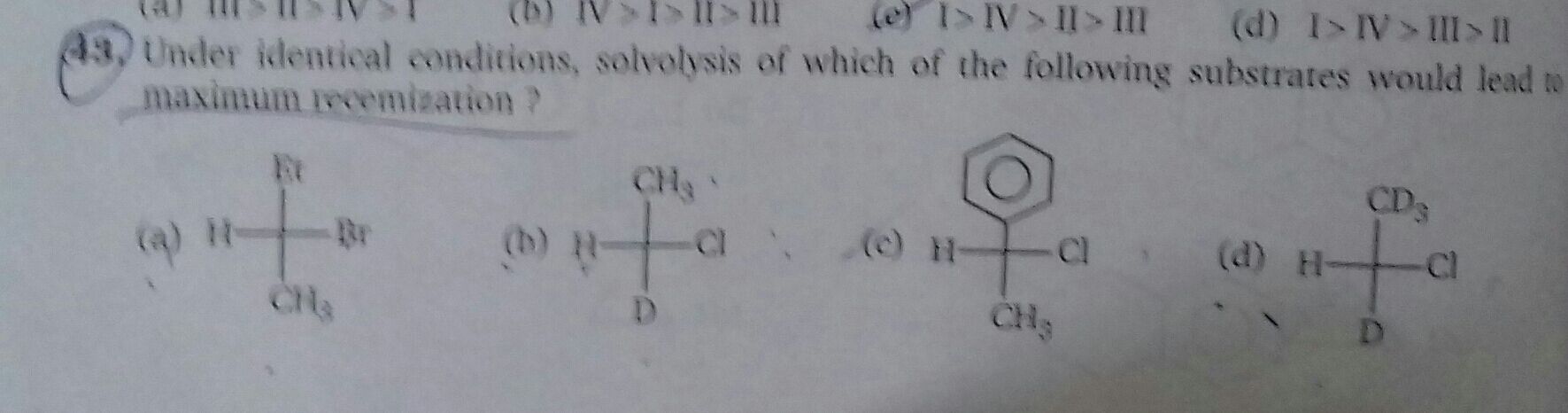JEE Class main Answered
Which of the following is soluble in water
a) CS2, b) C2H5OH c) CCl4, d) CHCl3
My doubt is- why CHCl3 is not soluble, it is polar and according to like dissolves like, it should dissolve in polar solvent like water. Does for soluble in water 'H- bonding' is necessary.
Asked by srkgb8018 | 10 May, 2024, 07:36: AM
Dear Student,
Water is a polar covalent compound.
Ethanol is also a polar covalent compound.
Chloroform is not soluble in water because of the absence of H bonding.
With water, hydrogen bonds are formed only in the case of F, O, and N, as the difference in electronegativity between H and F, O & N is very high.
The difference in electronegativity is not much between H and Cl. So, H bonding is not possible between chloroform and water.
Answered by | 12 May, 2024, 12:24: PM
JEE main - Chemistry
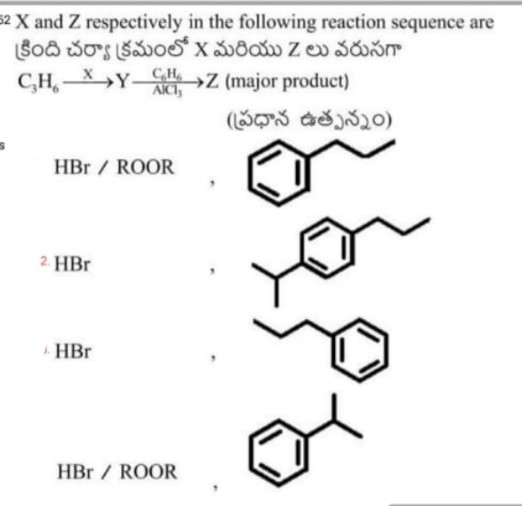
Asked by amanniju2 | 15 May, 2024, 09:05: AM
JEE main - Chemistry
Asked by srkgb8018 | 10 May, 2024, 07:36: AM
JEE main - Chemistry
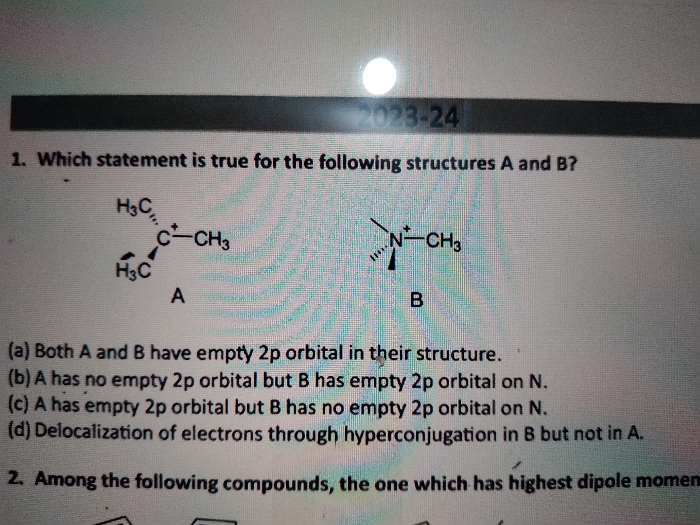
Asked by jacksparrow7352133590 | 09 May, 2024, 20:20: PM
JEE main - Chemistry
Asked by patelamrutbhaib24 | 28 Jan, 2024, 13:44: PM
JEE main - Chemistry
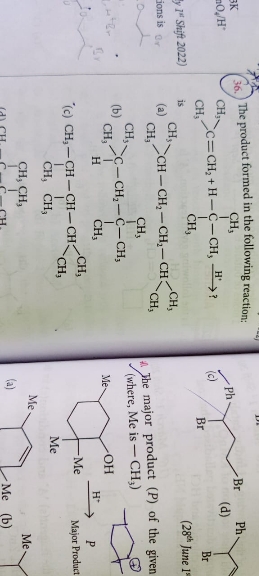
Asked by harshakancharla329 | 27 Jan, 2024, 09:07: AM
JEE main - Chemistry
Asked by mp797056 | 23 Oct, 2023, 16:23: PM
JEE main - Chemistry
Asked by dheerajrao2005 | 26 Mar, 2022, 19:06: PM
JEE main - Chemistry
Asked by yasharthshankar | 05 Jun, 2020, 23:39: PM