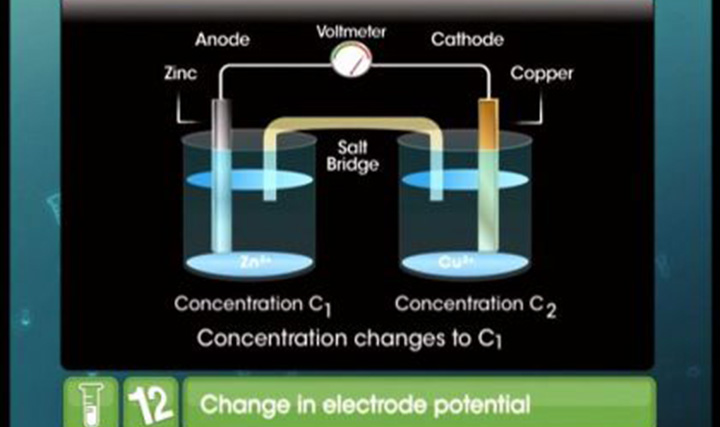
CBSE Class 12-science Chemistry Nernst Equation
What is the significance of the Nernst equation? What does it tell you about the reduction potential of an electrochemical reaction? Get the answers for these important questions with TopperLearning’s CBSE Class 12 Science Chemistry Electrochemistry – Nernst Equation study materials. Understand the application of the Nernst equation by listening to our Chemistry expert’s video lectures on our learning portal.
In your CBSE Class 12 Science Chemistry board exam, you may find questions based on the Nernst equation. To be able to answer your exam questions, you can build your Chemistry skills with our online learning resources. These include topic notes, MCQs, sample practice papers and more.
-
46,47

- Can you upload notes on a nernst equations explanation
- Q. 6. Calculate the emf of the following cell at 298 K Cr(s)/Cr³+ (0.1M)//Fe²+ (0.01M)/Fe(s) [Given: Eºcell = + 0.30 V]
-
Please answer this question.
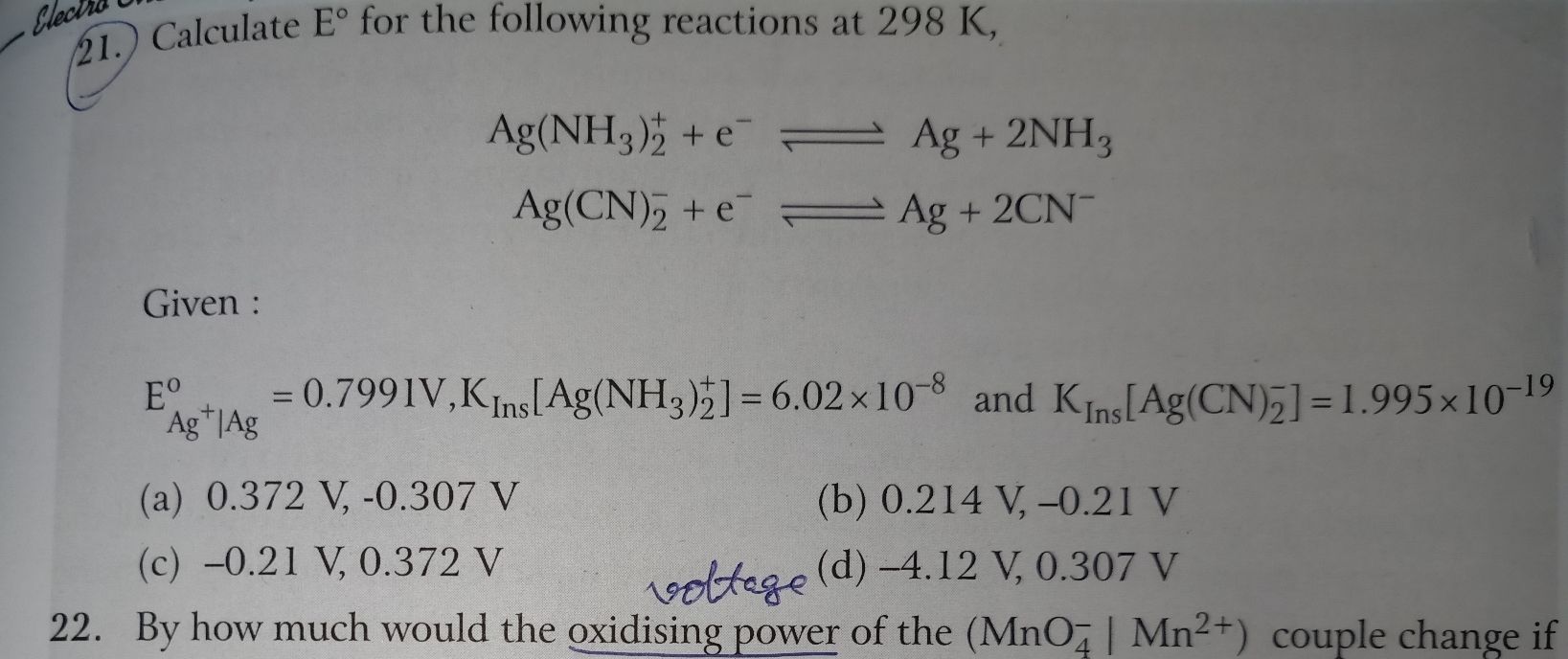
- For what concentration of Ag+ will the EMF of te given cell be zero at 25 C .If the concentration of Cu2+ is 0.1 M. EoAg+ = 0.80v and Eo Cu2+ = 0.34 V
-
Please answer.

- Electrochemistry What's 'n' in the equation: E°(cell) = 0.0591/n log Kc
- Predict whether the following redox reaction is feasible under the standard conditions or not. Sn2+(aq) + Cu(s) → Sn(s) + Cu2+(aq)
- Zn(s) | Zn2+(0.01M) || Pb2+(1.OM)|Pb(s) Given: Eo Pb2+/Pb = -0.12V and Eo Zn2+/Zn = -0.76V, what is the cell potential of the cell?
- Calculate the reduction potential for the following half cell reaction at 298 K. Ag+(aq) + e- → Ag(s) Given that (Ag+) = )0.1M and E° = + 0.80V