CBSE Class 12-science Chemistry Electrochemical Cells
Study CBSE Class 12 Science Chemistry Electrochemistry – Electrochemical Cells with TopperLearning’s concept videos by experts. Our Chemistry expert makes the chapter concepts interesting with simplified explanations and supporting visuals. Quickly go through concepts like redox reactions, electrochemical cells and galvanic cells by reading our Chemistry topic notes.
Revise CBSE Class 12 Science Chemistry questions and answers with our previous years’ papers and solutions. In addition, practise electrochemistry-based questions with our sample question papers. Our online practice tests and self-assessments will further benefit you with gaining conceptual clarity. All our learning materials are quickly accessible online through our study portal 24/7.
-
consider the following reaction

- what is electrochemical cell
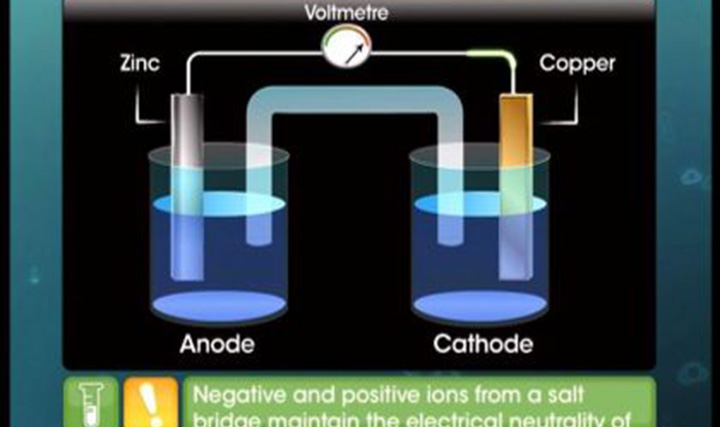
- what is galvanic cell
- the electric charge present on di positive magnesium ion is
- How to make simple equations for anode and cathode in standard electrode potential? How to understand their sign if the value of reduction and oxidation in questions?
- in an electrochemical cell
- Calculate the no. Of coulpmb required to deposit 40.5g AL when the electrode rxn is AL3+ +3e--->AL
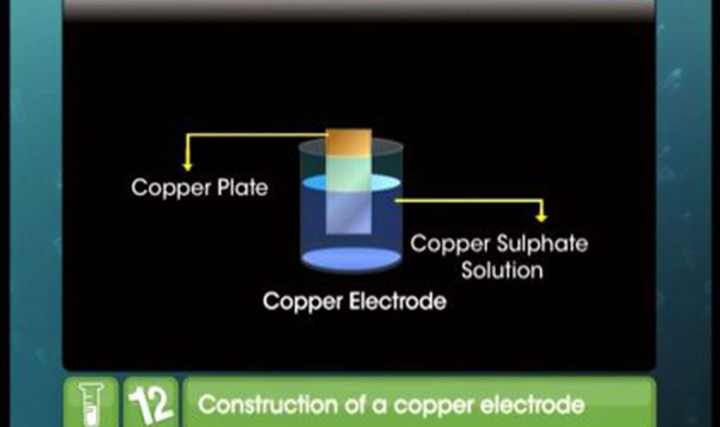
- when zinc plate is dipped into a blue coloured solution of cuso4 then its colour becomes white why
- cell constant G*= l /A Here A stands for area of Electrode or Electrolytic solution
- What is galvanic cell?