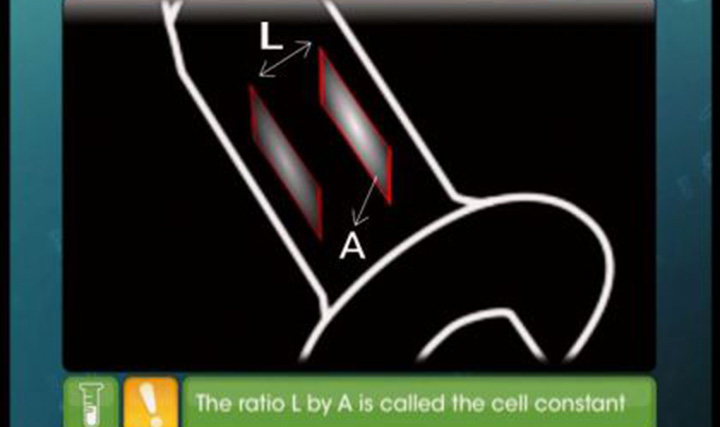
CBSE Class 12-science Chemistry Kohlrausch's Law
Revise Kohlrausch’s law of independent migration of ions with the assistance of the CBSE Class 12 Science Chemistry Electrochemistry study materials at TopperLearning. Use our video lessons, topic notes and textbook solutions to study this important law in Chemistry. These Chemistry learning resources are available online 24/7 so that you can study at your convenience.
In addition, our CBSE Class 12 Science Chemistry sample papers and solutions are developed by Chemistry experts to help you with mock exams. You can also practise Chemistry questions based on Kohlrausch’s law in the previous years’ question papers which are available on our study portal.
- how to identify cathode and anode in chemical reaction when only electrode potential is given?
- what is kohlrausch law? write it's applications.
- Calculate ^°for weak electrolyte by the help of kohlrausch law
-
give me clear refans

-
yfh

- Molar conductance values at infinite dilution of Na+ and Cl- ions are 50.11*10^-4Sm^2mol^-1 and 76.34*10^-4Sm^2mol^-1 respectively. Calculate the transport number of Na+ ion.
- The equivalent conductance of a decimolar solution of acetic acid was found to be 1.58*10^-3Sm^2eq^-1 at a given temperature. Calculate the degree of dissociation of acetic acid.
- How to calculate the antilog of 35 ?
- What is the major application of Kohlrausch's Law.
- Why each ion at infinite makes a unique contribution towards molar conductivity?