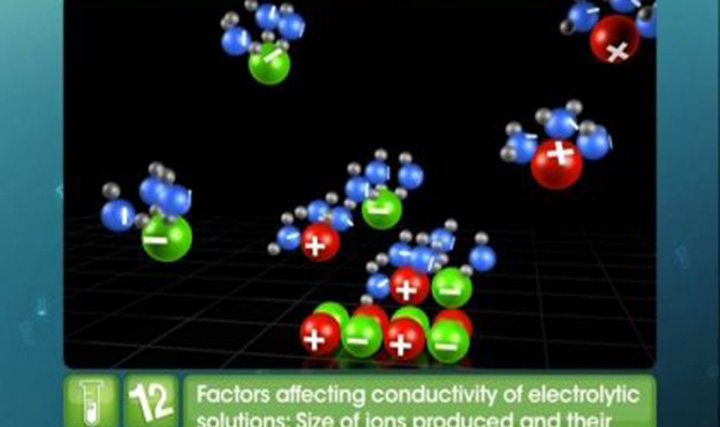
CBSE Class 12-science Chemistry Conductivity of Solutions
Learn online for your Chemistry board exam with CBSE Class 12 Science Chemistry Electrochemistry – Conductivity of Solutions. Study the factors affecting the conductivity of electrolytic solutions with our online video lessons. These video lessons include the important concepts covered in the latest Chemistry syllabus.
Revise electrolytic conductance with TopperLearning’s CBSE Class 12 Science Chemistry topic notes. If you have the exam blues, practise the important Chemistry questions and answers using our textbook solutions and solved sample papers. Our learning materials are designed to assist you in getting over your exam fear and gaining the confidence to attempt questions successfully in your Chemistry exam.
- establish the relation between molar conductance and specific conductance of a solute.
- what is molar conductivity
- How many gram of Cu will be obtained by passing 4.5 ampere current thrugh 1 litre 0.6M CuCl2 aqueous solution by dipping inert electrodes for 1 hour? what will be the change in concentration ?
- How f2 have more oxidising power than cl2
-
3.66

-
3.13

-
Plz answer 3.14

- Why does specific conductivity decrease with dilution
- Define Ohm's law?
- What is the relation between degree of ionisation and dilution of weak electrolytes?