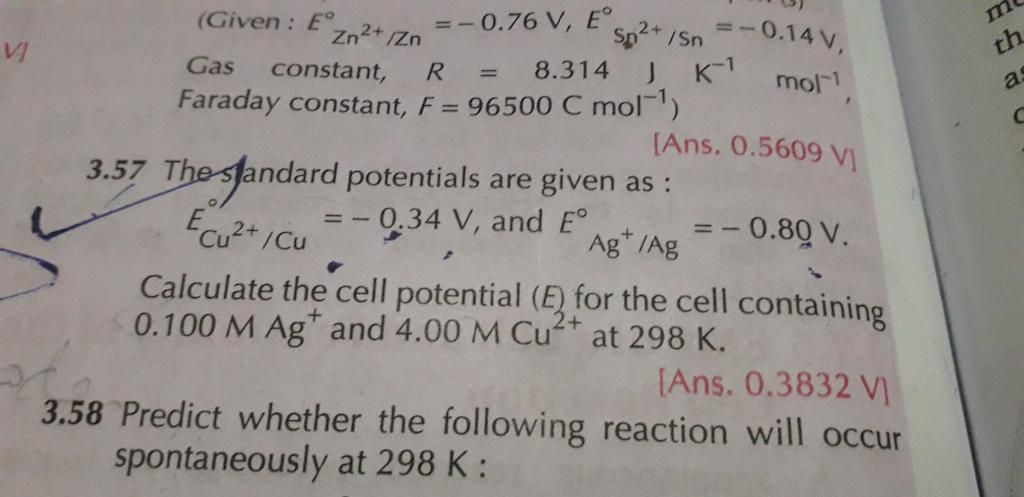CBSE Class 12-science Chemistry Electrolysis
Improve your board exam score with CBSE Class 12 Science Chemistry Electrochemistry – Electrolysis video lessons available at TopperLearning. Our experienced Chemistry expert explains the concept of Electrolysis using attractive visuals in our concept videos. These video lessons will help you get a good grip on topics like Faraday’s laws of electrolysis. Once your concepts of Electrochemistry are clear, you can write accurate answers in your exam and score more marks.
For exam-focused revision, we have CBSE Class 12 Science topic notes, sample papers, practice tests and more. The previous years’ question papers on our study portal should also help you in getting ready for your Class 12 Chemistry exam.
- Electrolysis of concentrated H2SO4 and diluted H2SO4
- Electrochemistry:- Comment on pH of solution after electrolysis of ZnSO4 in aqueous media by using Zn electrode.
- Aluminium is extracted by electrolysis of
-
Pls explain
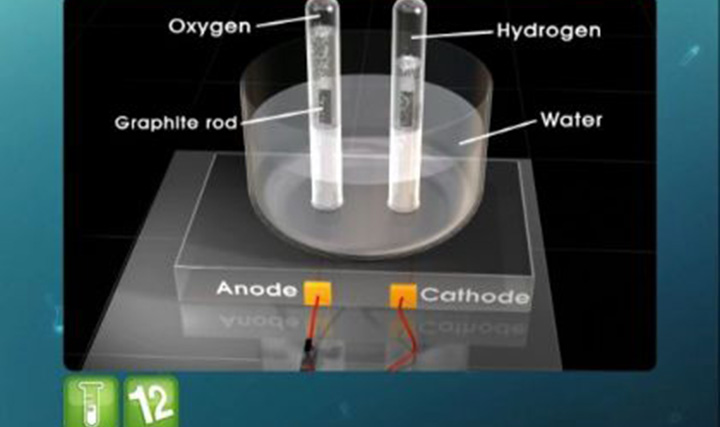
- TOTAL VOLUME OF GASES EVOLVED AT STP WHEN 36g OF H2O ARE COMPLETELY ELECTROLYSED BETWEEN PLATINUM ELECTRODES.
- What happens to flow of electrons if we introduce two anodes at one end of the cathode
-
3.66 sum plz

-
3.71 plz

-
3.52 numerical plz
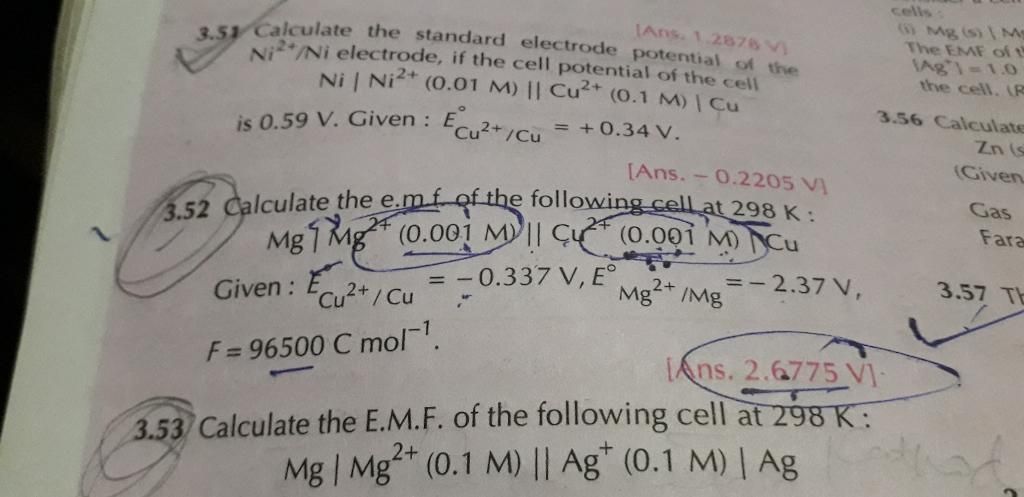
-
3.57 plz