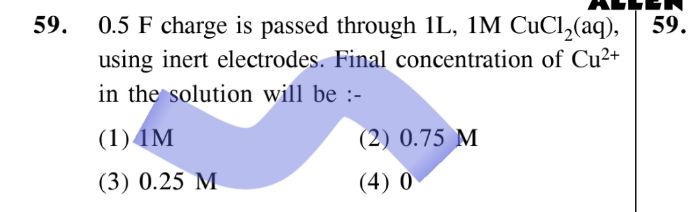
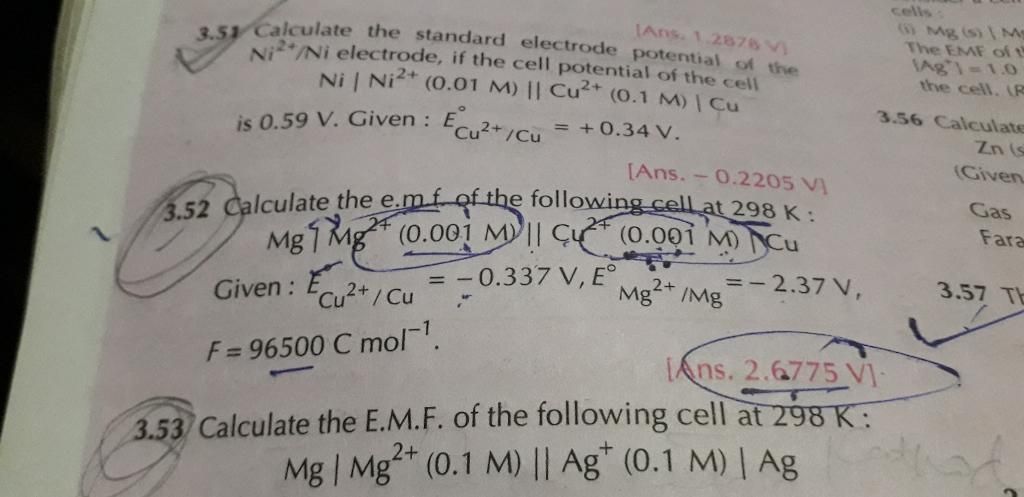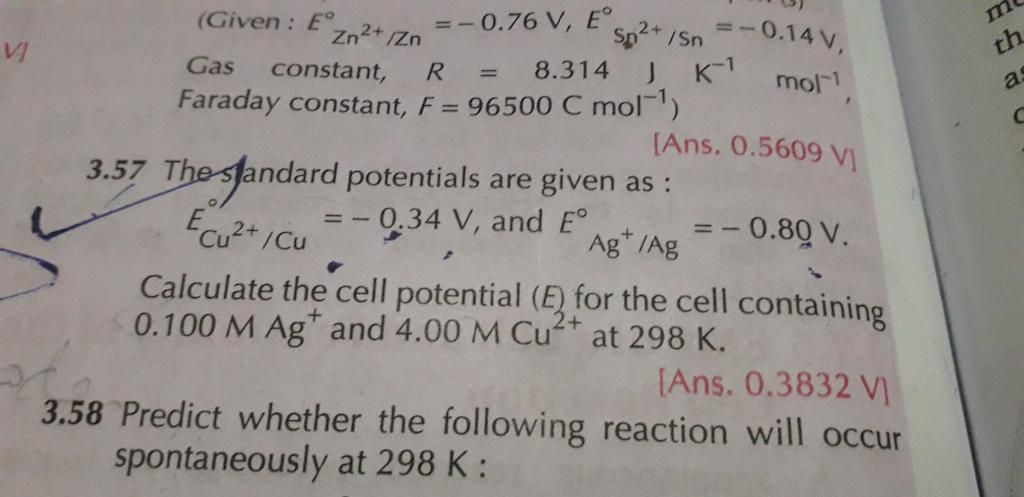CBSE Class 12-science Answered
Electrochemistry:-
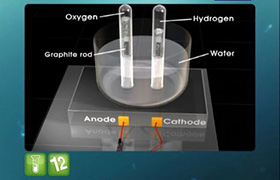
Comment on pH of solution after electrolysis of ZnSO4 in aqueous media by using Zn electrode.
Asked by avaneesh5116 | 07 Aug, 2020, 17:24: PM
Zinc is having lower standard reduction potential than hydrogen, so at anode oxidation of zinc takes place and evolution of hydrogen gas takes place at the cathode. Due to absence of H+ and presence of OH- ions in solutions, pH increases.
Answered by Ravi | 07 Aug, 2020, 21:20: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by routraypriyanka255 | 04 Jun, 2024, 23:43: PM
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 17:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 16:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 20:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 21:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:11: PM