CBSE Class 12-science Answered
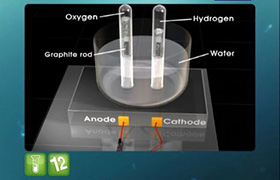
What happens to flow of electrons if we introduce two anodes at one end of the cathode
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 21:53: PM
In electrons if we are using two anodes and one cathode, then number of electrons will be increased in solution but cathode is one that accepts electron so electrons will be produced in more quantity but accepted by cathode in same quantity per unit time so electron flow will be same but total number of electrons will be more that will not contribute to producing electric current.
Answered by Ravi | 02 Nov, 2019, 17:28: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by routraypriyanka255 | 04 Jun, 2024, 23:43: PM
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 17:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 16:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 20:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 21:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:11: PM