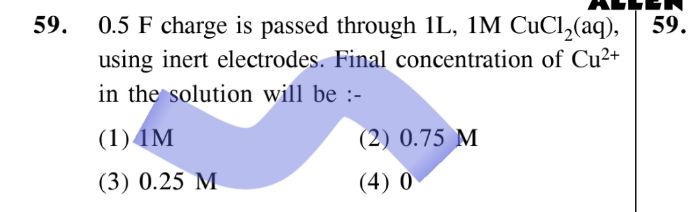
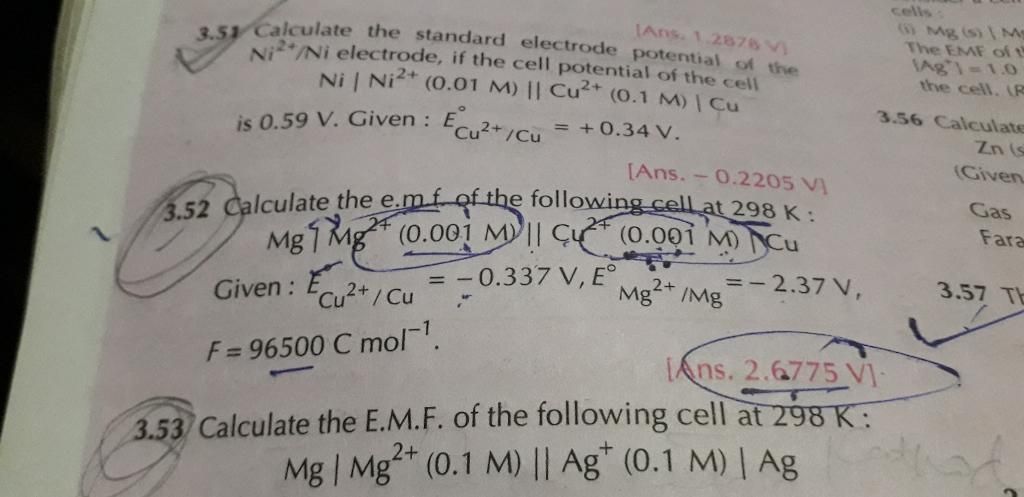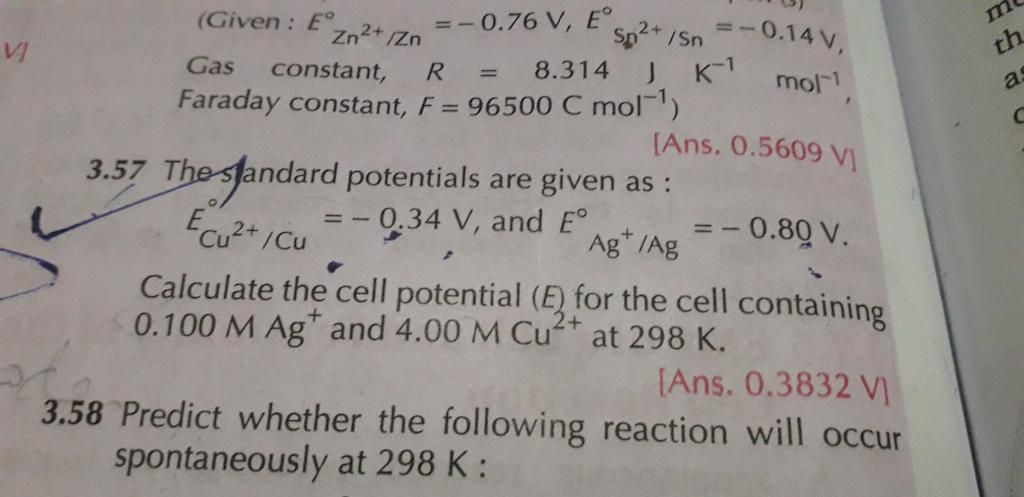CBSE Class 12-science Answered
3.71 plz

Asked by lovemaan5500 | 19 Aug, 2019, 23:13: PM
Given:
I = 25 ampere
Volume = 20 L
Mass of Cl2 = 1 kg
= 1000 gm
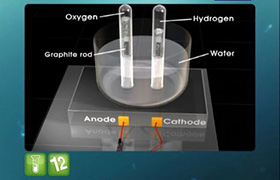
(i) Reactions taking place at electrodes:
Cathode: 2H2O + 2e− → H2(g) + 2OH−(aq)
Anode : 2Cl−(aq) → Cl2 + 2e−
(ii)
Mole of Cl2
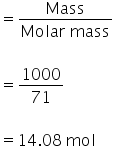
Electrons needed to carry out electrolysis = 2 × 14.08
= 28.16 mol
Quantity of electricity carried by these electrons = 28.16 × 96500 C
Time required,
Current efficiency is 62%
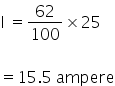

(iii) Molarity of solution:
Amount of OH− ions released = 2× 14.08
= 28.16 mol
Molarity of OH− ions
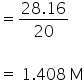
Answered by Varsha | 20 Aug, 2019, 11:06: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by routraypriyanka255 | 04 Jun, 2024, 23:43: PM
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 17:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 16:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 20:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 21:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:11: PM