CBSE Class 12-science Answered
3.57 plz
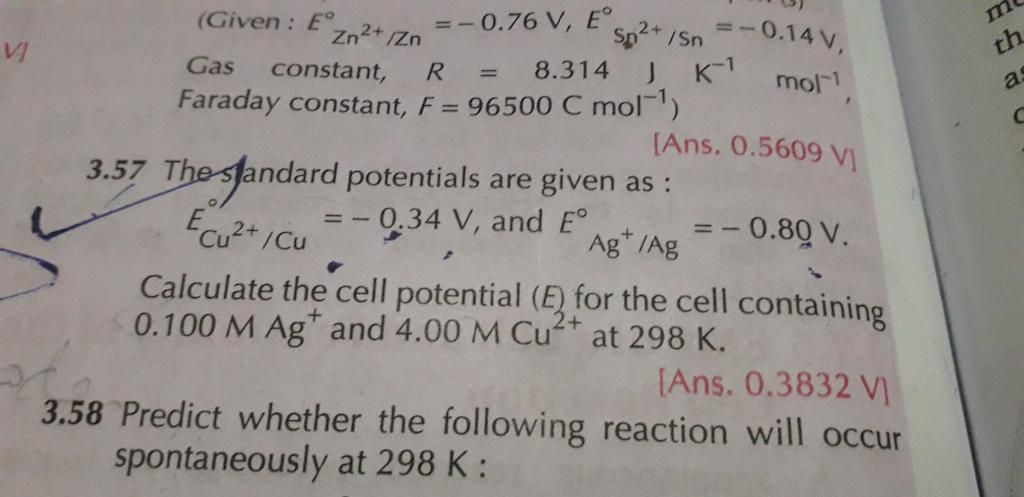
Asked by lovemaan5500 | 19 Aug, 2019, 23:11: PM
Given:
E0Cu2+/Cu = 0.34 V
E0Ag2+/Ag = 0.80 V
R = 8.314 JK− mol−
F = 96500 C/mol
E0cell = E0cathode −E0anode
= 0.80 − 0.34
= 0.46 V
The net cell reaction,
Cu + 2Ag+ → Cu+ + 2Ag
n = 2
Nernst equation,
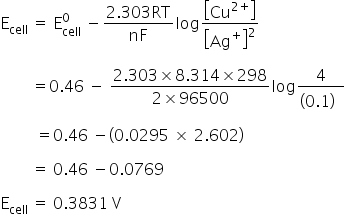
Cell potential is 0.3831 V
Answered by Varsha | 20 Aug, 2019, 15:49: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by routraypriyanka255 | 04 Jun, 2024, 23:43: PM
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 17:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 16:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 20:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 21:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:11: PM