CBSE Class 12-science Answered
3.66 sum plz

Asked by lovemaan5500 | 19 Aug, 2019, 23:14: PM
Given:
Mass of nitrobenzene = 12.3 gm
Current efficieny = 50%
Potetial V = 3 V
Energy = ?
Reduction reaction is,
C6H5NO2 +6H+ + e− → C6H5NH2 + 2H2O
Moles of C6H5NO2 to be reduced
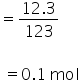
According to the equation, 0.1 mole of C6H5NO2 will require 0.6 mole of electrons. But efficiecy of current is 50%.
Therefore, 0.1 mole of C6H5NO2 will require 1.2 mole of electrons.
Electricity carried by 1.2 mol of electrons = 1.2 × 96500
= 115800 C
Answered by Varsha | 20 Aug, 2019, 11:40: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by routraypriyanka255 | 04 Jun, 2024, 23:43: PM
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 17:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 16:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 20:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 21:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 23:11: PM