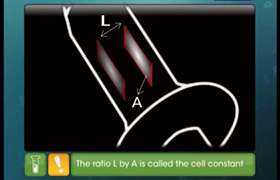
CBSE Class 12-science Answered
The equivalent conductance of a decimolar solution of acetic acid was found to be 1.58*10^-3Sm^2eq^-1 at a given temperature. Calculate the degree of dissociation of acetic acid.
Asked by mrudulmahadev1311 | 21 Aug, 2019, 12:17: PM
Equivalent conductance of acetic acid = 1.58 ×10−3 Sm2eq−1
Molar conductance of actic acid
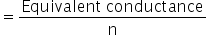
For acetic acid, n = 1
Molar conductance of actic acid = 1.58 ×10−3 Sm2eq−1
Limiting molar conductivity for acetic acid is,
Λ∞ =
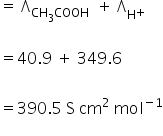
We know,
Degree of dissociation is,
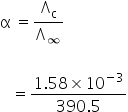
= 4.04 ×10−6
Answered by Varsha | 22 Aug, 2019, 02:55: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by samskruthikrishn | 12 Jan, 2024, 10:11: AM
CBSE 12-science - Chemistry
Asked by 2507king2006 | 03 Oct, 2023, 07:12: AM
CBSE 12-science - Chemistry
Asked by eyemunshah | 20 Nov, 2021, 11:19: PM
CBSE 12-science - Chemistry
Asked by samisiddiqui013 | 29 May, 2021, 07:49: PM
CBSE 12-science - Chemistry
Asked by mrudulmahadev1311 | 22 Aug, 2019, 07:47: AM
CBSE 12-science - Chemistry
Asked by mrudulmahadev1311 | 21 Aug, 2019, 12:17: PM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 26 May, 2018, 05:06: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2014, 10:56: AM