CBSE Class 12-science - Kohlrausch's Law Videos
Kohlrausch's Law
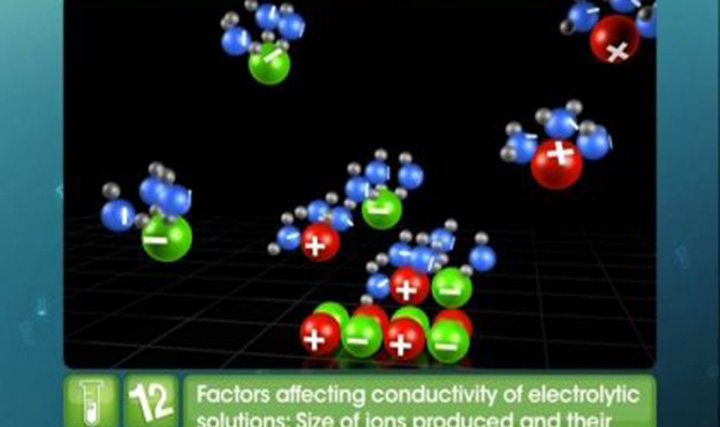
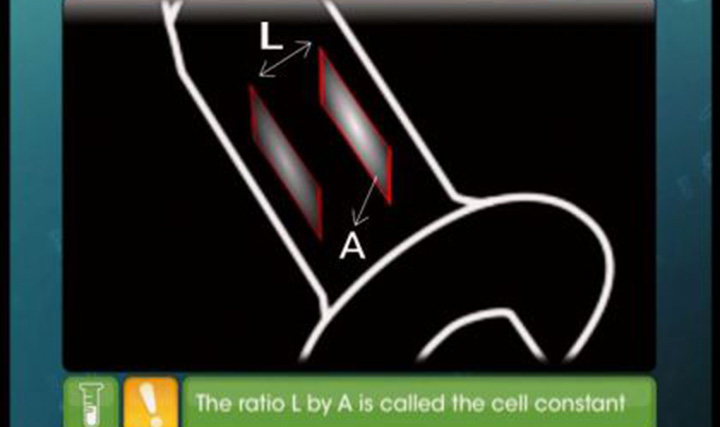
This video explains comparison and calculation of conductivities of strong and weak electrolytes, Kholrausch's law and its application.
More videos from this chapter
View All- how to identify cathode and anode in chemical reaction when only electrode potential is given?
- what is kohlrausch law? write it's applications.
- Calculate ^°for weak electrolyte by the help of kohlrausch law
-
give me clear refans

-
yfh

- Molar conductance values at infinite dilution of Na+ and Cl- ions are 50.11*10^-4Sm^2mol^-1 and 76.34*10^-4Sm^2mol^-1 respectively. Calculate the transport number of Na+ ion.
- The equivalent conductance of a decimolar solution of acetic acid was found to be 1.58*10^-3Sm^2eq^-1 at a given temperature. Calculate the degree of dissociation of acetic acid.
- How to calculate the antilog of 35 ?
- What is the major application of Kohlrausch's Law.
- Why each ion at infinite makes a unique contribution towards molar conductivity?