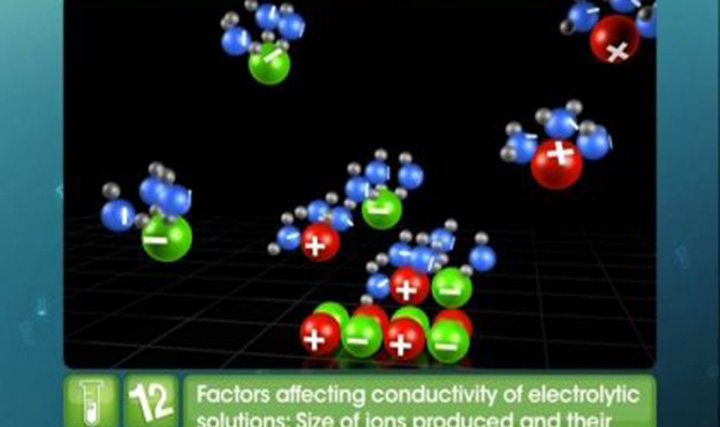
CBSE Class 12-science - Electrochemical Cells Videos
Electrochemical Cells
This video explains the meaning of Redox reaction, construction and working of Galvanic cells and salt bridge
More videos from this chapter
View All-
consider the following reaction

- what is electrochemical cell
- what is galvanic cell
- the electric charge present on di positive magnesium ion is
- How to make simple equations for anode and cathode in standard electrode potential? How to understand their sign if the value of reduction and oxidation in questions?
- in an electrochemical cell
- Calculate the no. Of coulpmb required to deposit 40.5g AL when the electrode rxn is AL3+ +3e--->AL
- when zinc plate is dipped into a blue coloured solution of cuso4 then its colour becomes white why
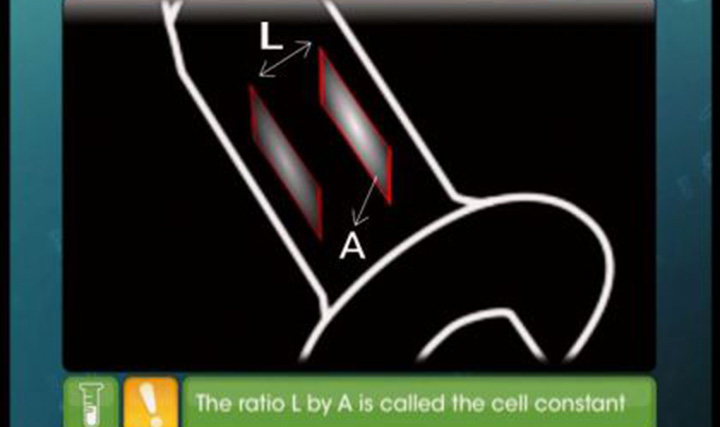
- cell constant G*= l /A Here A stands for area of Electrode or Electrolytic solution
- What is galvanic cell?