CBSE Class 12-science Answered
What is galvanic cell?
Asked by kripanjalihimansu | 28 Feb, 2019, 06:57: AM
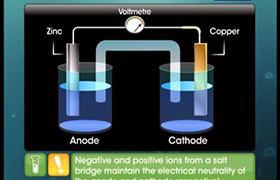
Galvanic cell:
A galvanic cell is an electrochemical cell that converts the chemical energy of a spontaneous redox reaction into electrical energy.
- In this device, the Gibbs energy of the spontaneous redox reaction is converted into electrical work
which may be used for running motor or other electrical gadgets like heater, fan, geyser, etc.
- The following redox reaction occurs in Galvanic cell,

Answered by Varsha | 28 Feb, 2019, 11:04: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by anithaanu629940 | 11 May, 2024, 12:31: PM
CBSE 12-science - Chemistry
Asked by summiafroz31 | 06 Feb, 2024, 20:39: PM
CBSE 12-science - Chemistry
Asked by aryamankrsinha2002 | 29 Nov, 2023, 11:39: AM
CBSE 12-science - Chemistry
Asked by banneramadevi | 26 Jul, 2023, 20:51: PM
CBSE 12-science - Chemistry
Asked by Poojanisha1988 | 19 Jul, 2023, 21:59: PM
CBSE 12-science - Chemistry
Asked by jajimuji2306 | 03 Apr, 2022, 13:38: PM
CBSE 12-science - Chemistry
Asked by Harshfarwaha | 23 Jul, 2020, 15:27: PM
CBSE 12-science - Chemistry
Asked by sourabhkumar9923 | 19 May, 2020, 20:21: PM
CBSE 12-science - Chemistry
Asked by ssharondaniel | 27 Jul, 2019, 18:22: PM
CBSE 12-science - Chemistry
Asked by kripanjalihimansu | 28 Feb, 2019, 06:57: AM