CBSE Class 12-science Answered
Dear Student,
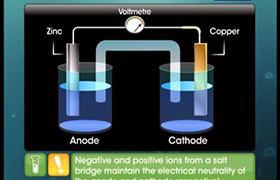
- In an indirect redox reaction, the device that converts chemical energy into electrical energy is known as an electrochemical cell.
- In an electrochemical cell:
- The half-cell in which oxidation takes place is known as the oxidation half-cell
- The half cell in which reduction takes place is known as the reduction half cell.
- Oxidation takes place at the anode which is negatively charged and reduction takes place at the cathode which is positively charged.
- Transfer of electrons takes place from anode to cathode while electric current flows in the opposite direction.
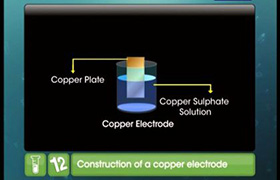
- An electrode is made by dipping the metal plate into the electrolytic solution of its soluble salt.
- A salt bridge is a U-shaped tube containing an inert electrolyte in agar-agar and gelatine.
3. A salt bridge maintains electrical neutrality and allows the flow of electric current by completing the electrical circuit.
4. Representation of an electrochemical cell:
a. Anode is written on the left while the cathode is written on the right.
b. Anode represents the oxidation half cell and is written as:
Metal/Metal ion (Concentration)
c. Cathode represents the reduction of half cell and is written as:
Metal ion (Concentration)/Metal
d. Salt bridge is indicated by placing double vertical lines between the anode and the cathode
e. Electrode potential is the potential difference that develops between the electrode and its electrolyte. The separation of charges at the equilibrium state results in the potential difference between the metal and the solution of its ions. It is the measure of the tendency of an electrode in the half-cell to lose or gain electrons.