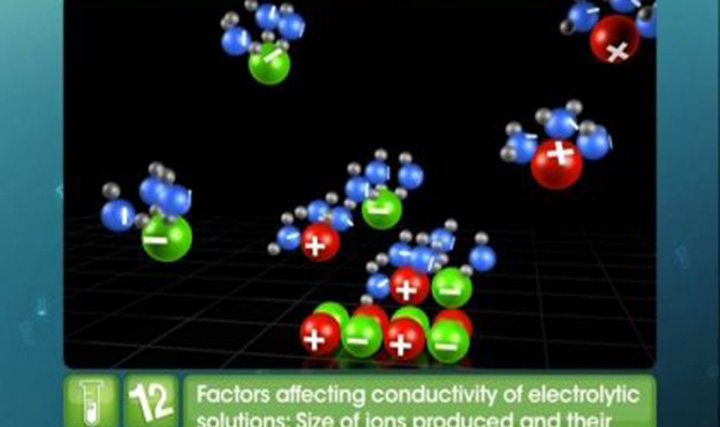
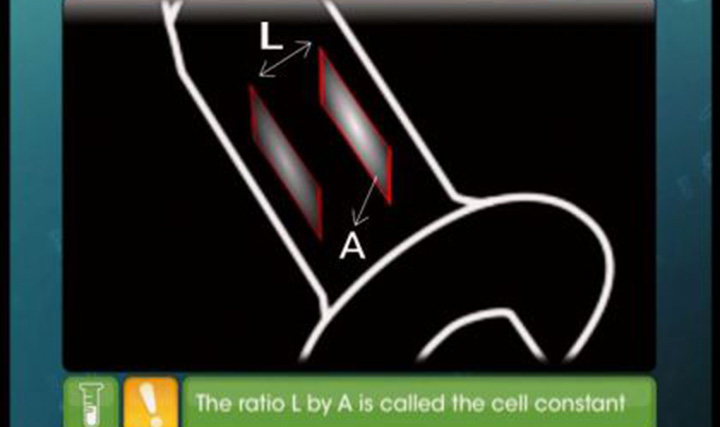
CBSE Class 12-science - Nernst Equation Videos
Nernst Equation
This video explains Nernst equation, Cell Potential, Equilibrium Constant, Gibb's Energy
More videos from this chapter
View All-
46,47

- Can you upload notes on a nernst equations explanation
- Q. 6. Calculate the emf of the following cell at 298 K Cr(s)/Cr³+ (0.1M)//Fe²+ (0.01M)/Fe(s) [Given: Eºcell = + 0.30 V]
-
Please answer this question.
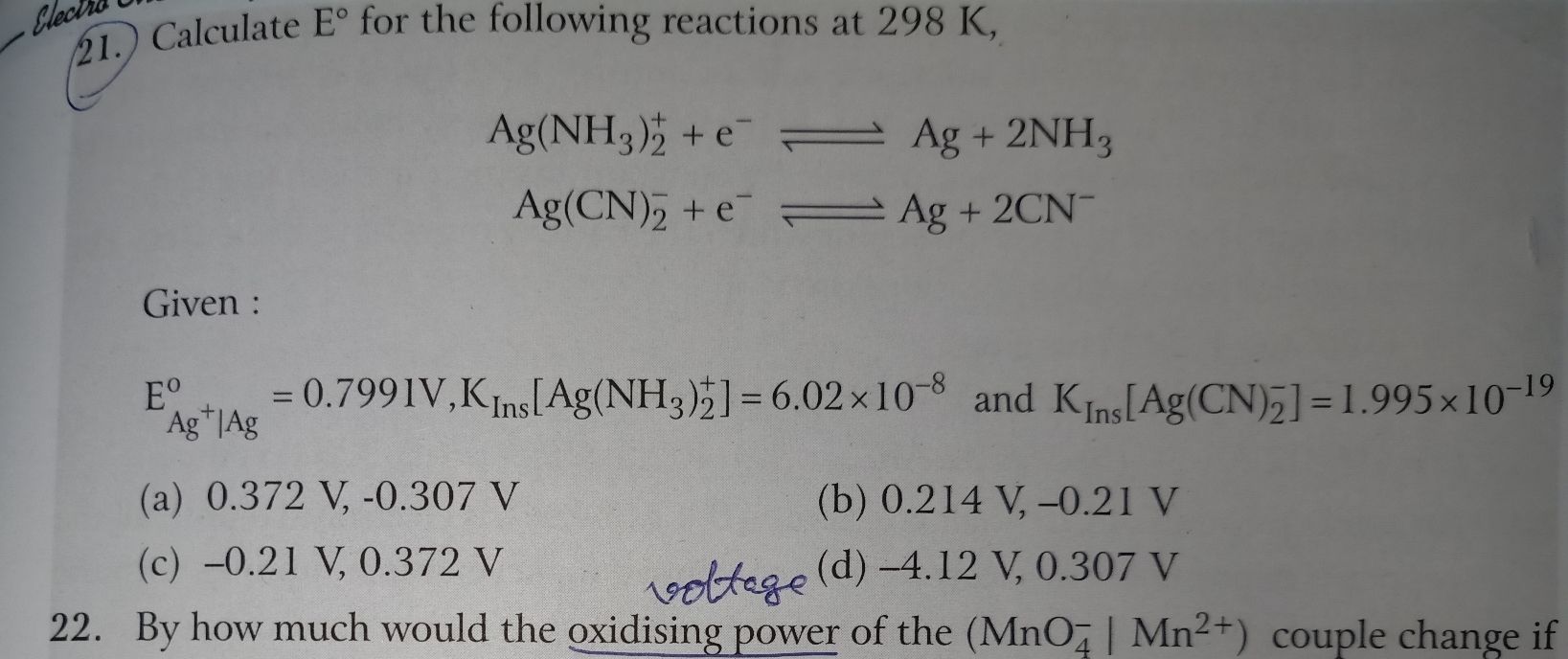
- For what concentration of Ag+ will the EMF of te given cell be zero at 25 C .If the concentration of Cu2+ is 0.1 M. EoAg+ = 0.80v and Eo Cu2+ = 0.34 V
-
Please answer.

- Electrochemistry What's 'n' in the equation: E°(cell) = 0.0591/n log Kc
- Predict whether the following redox reaction is feasible under the standard conditions or not. Sn2+(aq) + Cu(s) → Sn(s) + Cu2+(aq)
- Zn(s) | Zn2+(0.01M) || Pb2+(1.OM)|Pb(s) Given: Eo Pb2+/Pb = -0.12V and Eo Zn2+/Zn = -0.76V, what is the cell potential of the cell?
- Calculate the reduction potential for the following half cell reaction at 298 K. Ag+(aq) + e- → Ag(s) Given that (Ag+) = )0.1M and E° = + 0.80V