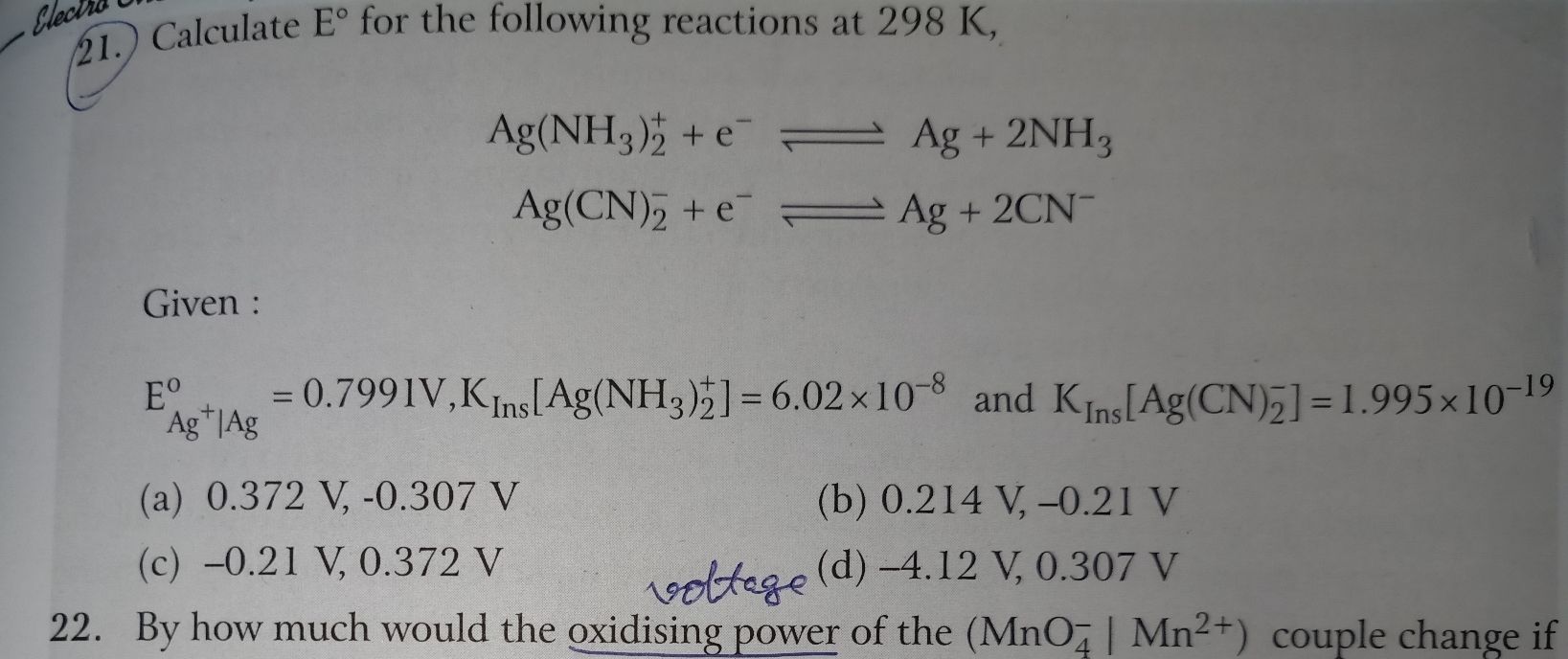CBSE Class 12-science Answered
Predict whether the following redox reaction is feasible under the standard conditions or not. Sn2+(aq) + Cu(s) → Sn(s) + Cu2+(aq)
Asked by Topperlearning User | 22 Jun, 2016, 14:42: PM
In the given cell the reduction of Sn 2+ ions takes pace and also the oxidation of copper metal to copper ions takes place.Eo cell is
E°cell = E°cathode - E°anode
E°cell = E° Sn2+ / Sn - E° Cu2+ / Cu
= -0.14 V – ( -0.34) = 0.20V
Δ Go = - nFEo celll
= -2 x 96500 x 0.20 V = -38600 J
Answered by | 22 Jun, 2016, 16:42: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by yashwanthgowdakn4 | 22 Feb, 2024, 21:14: PM
CBSE 12-science - Chemistry
Asked by keerthana.d.cst.2022 | 22 Aug, 2023, 20:18: PM
CBSE 12-science - Chemistry
Asked by amankumar2004112 | 04 May, 2022, 13:12: PM
CBSE 12-science - Chemistry
Asked by Balbir | 02 Oct, 2019, 00:29: AM
CBSE 12-science - Chemistry
Asked by Jsiajaijiaia | 27 Aug, 2019, 00:23: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 14 Aug, 2019, 00:46: AM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 19 May, 2018, 15:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 14:42: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 14:41: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 14:41: PM