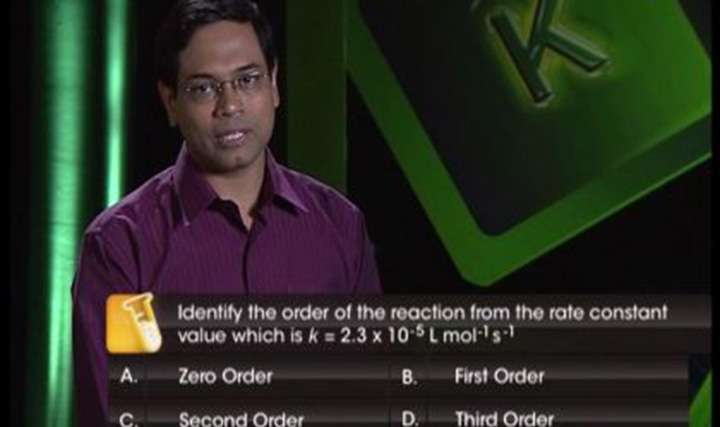CBSE Class 12-science Chemistry Order and Molecularity of Reaction
Revise concepts like elementary reactions and complicated reactions with our CBSE Class 12 Science Chemistry Chemical Kinetics – Order and Molecularity of Reaction videos. Our concept videos are engaging and will help in retention of learned concepts. Understand the difference between molecularity and order of reaction by reading through our Chemistry topic notes.
Do you want to watch videos and learn concepts? Or do you prefer writing and practising CBSE Class 12 Science Chemistry textbook solutions during revision? You will find everything you need to ace your Chemistry board exam at TopperLearning. To prepare for your board exam, you should also use our previous years’ Chemistry papers and solved sample papers.
-
relation between k and t for first order reaction

- Definition of zero order, 1st order and 2nd order reaction
- The half life for a first order reaction is 10 mins .What percentage of reactant will be left behind after 60mins.
-
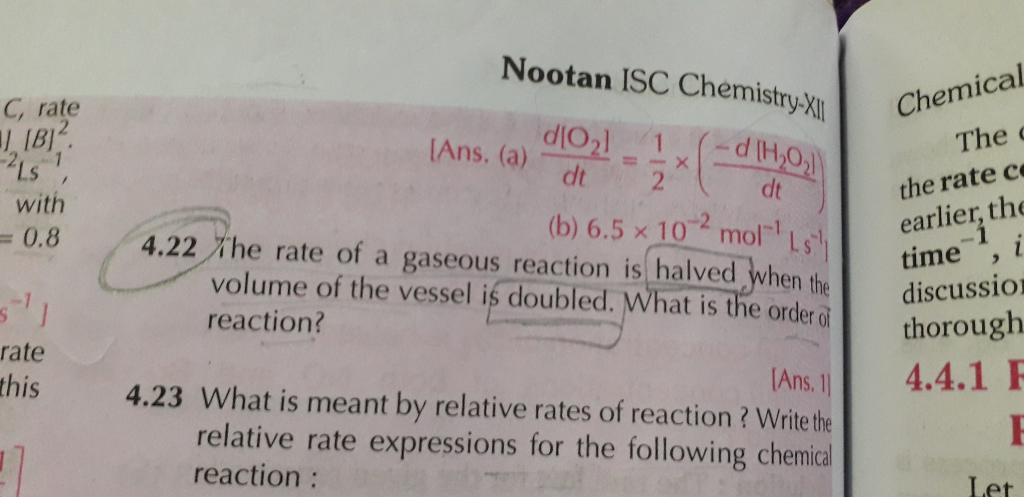
4.22 sum
-
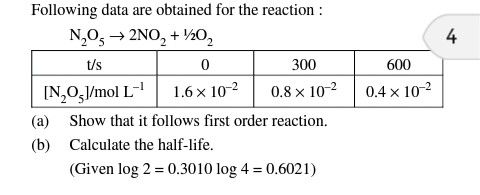
4.32 numerical plz

- Write a condition under which a bimolecular reaction is kinetically first order. Give an example of such a reaction
-
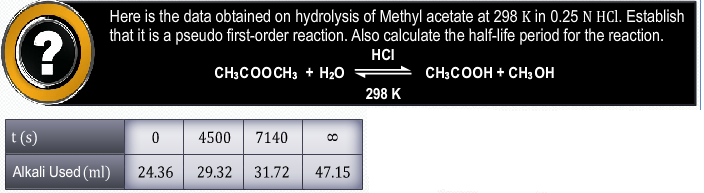
Pls answer
- what is the order of reaction if rate of reaction becomes 3 times and concentration of reaction becomes 9 times
-
please solve it
- The reaction 2NO2Cl → 2NO2 + Cl2 have the rate law: r = k [NO2Cl]. What is it mechanism?