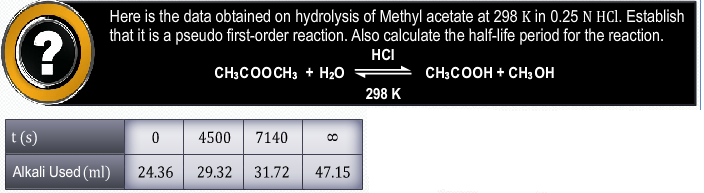
CBSE Class 12-science Answered
Pls answer
Asked by Vidya | 07 Mar, 2019, 19:27: PM
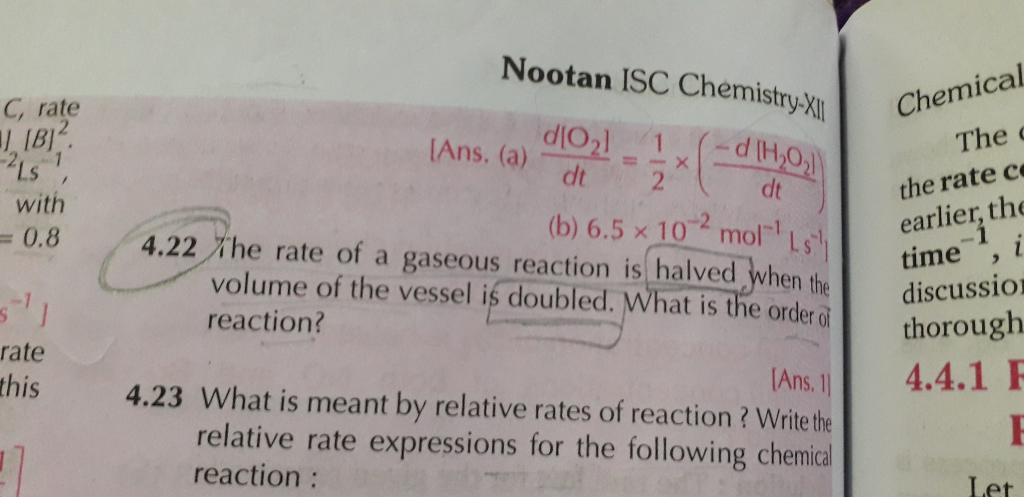
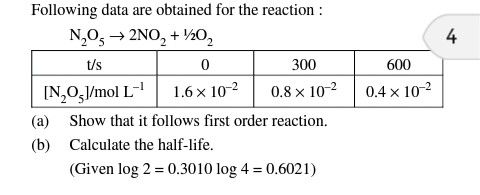
(a) It is a first order reaction.
From the given data,
At t = 300 sec
Initial concentration [A0] = 1.6 Χ 10-2 mol/lit
Final Concentration [A] = 0.8 Χ 10-2 mol/lit
We have,
The rate of reaction is
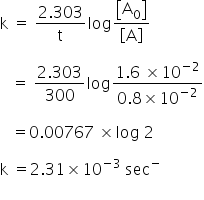
Now at t = 600 sec
Initial concentration [A0] = 0.8 Χ 10-2 mol/lit
Final Concentration [A] = 0.4 Χ 10-2 mol/lit
Rate of reaction is,
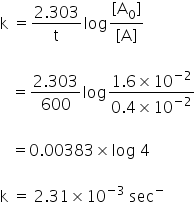
As the value of rate constant k is constant in both cases, it is the first order reaction.
(a) The half life of reaction,
For first-order reactions,
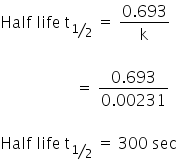
The half-life of the reaction is 300 sec.
Answered by Varsha | 08 Mar, 2019, 12:51: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by BABUYVU | 02 Jun, 2024, 09:30: AM
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by arunhys123 | 04 Jul, 2020, 19:36: PM
CBSE 12-science - Chemistry
Asked by dhruvrana348 | 28 Jun, 2020, 08:58: AM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 06 Jan, 2020, 15:39: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 18 Sep, 2019, 22:02: PM
CBSE 12-science - Chemistry
Asked by sanjeet.kumar | 12 Mar, 2019, 14:20: PM
CBSE 12-science - Chemistry
Asked by manpreetkaur19971993 | 10 Jan, 2019, 07:06: AM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 22 Jul, 2018, 20:27: PM