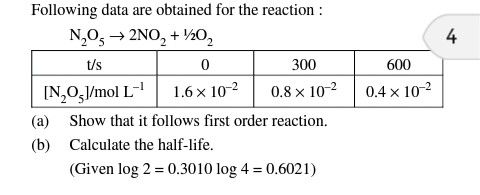
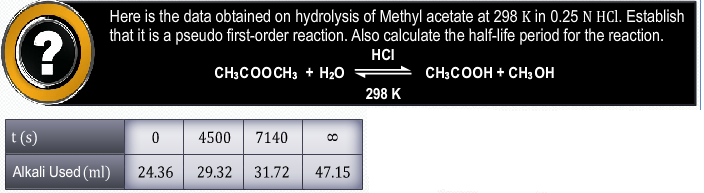
CBSE Class 12-science Answered
what is the order of reaction if rate of reaction becomes 3 times and concentration of reaction becomes 9 times
Asked by manpreetkaur19971993 | 10 Jan, 2019, 07:06: AM
We know that,
Rate = K[Reatant]n
[Reactant] = a
Rate = r1
r1 = K[a]n
It is given that rate of reaction becomes 3 time, and concentration of reaction becomes 9 times
Therefore,
3 r1 = K[9a]n
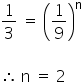
The order of reaction is 2.
Answered by Varsha | 10 Jan, 2019, 11:13: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by BABUYVU | 02 Jun, 2024, 09:30: AM
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by arunhys123 | 04 Jul, 2020, 19:36: PM
CBSE 12-science - Chemistry
Asked by dhruvrana348 | 28 Jun, 2020, 08:58: AM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 06 Jan, 2020, 15:39: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 18 Sep, 2019, 22:02: PM
CBSE 12-science - Chemistry
Asked by sanjeet.kumar | 12 Mar, 2019, 14:20: PM
CBSE 12-science - Chemistry
Asked by manpreetkaur19971993 | 10 Jan, 2019, 07:06: AM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 22 Jul, 2018, 20:27: PM