CBSE Class 12-science Answered
Write a condition under which a bimolecular reaction is kinetically first order. Give an example of such a reaction
Asked by sanjeet.kumar | 12 Mar, 2019, 14:20: PM
When one of the reactants is taken in excess so that its concentration hardly changes.
In such condition, the bimolecular reaction will be of first order.
Example:
Hydrolysis of ethyl acetate (0.01 mol) with 10 mol of water:
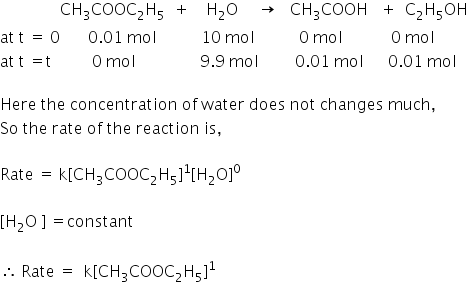
So in this reaction, the concentration of water is taken in excess and the bimolecular reaction behaves as first order reaction.
Answered by Varsha | 12 Mar, 2019, 19:53: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by BABUYVU | 02 Jun, 2024, 09:30: AM
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by arunhys123 | 04 Jul, 2020, 19:36: PM
CBSE 12-science - Chemistry
Asked by dhruvrana348 | 28 Jun, 2020, 08:58: AM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 06 Jan, 2020, 15:39: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 18 Sep, 2019, 22:02: PM
CBSE 12-science - Chemistry
Asked by sanjeet.kumar | 12 Mar, 2019, 14:20: PM
CBSE 12-science - Chemistry
Asked by manpreetkaur19971993 | 10 Jan, 2019, 07:06: AM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 22 Jul, 2018, 20:27: PM