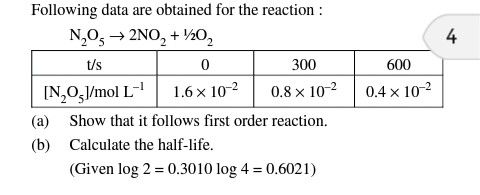
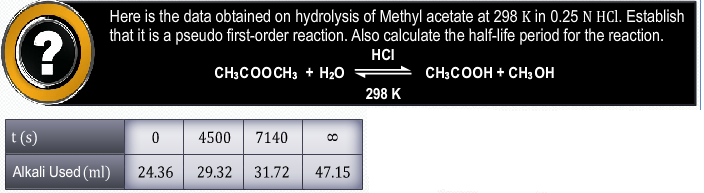
CBSE Class 12-science Answered
Definition of zero order, 1st order and 2nd order reaction
Asked by arunhys123 | 04 Jul, 2020, 19:36: PM
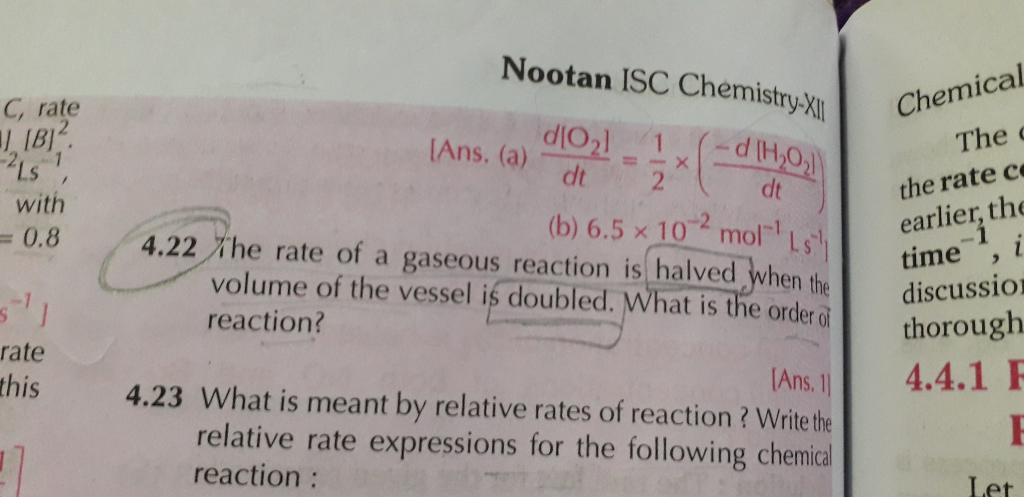
Zero Order Reaction- Reaction which proceeds at constant rate or reaction in which rate of reaction does not depend on concentration of reactants is called as xero order reaction.
First Order Reaction- Reaction in which rate of reaction depends linearly on only one reactant concentration is called as first order reaction.
Second Order Reaction- Reaction in which rate of reaction is proportional to the square of the concentration of a reactant or the product of the concentration of two reactants.
Answered by Ravi | 04 Jul, 2020, 21:41: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by BABUYVU | 02 Jun, 2024, 09:30: AM
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by arunhys123 | 04 Jul, 2020, 19:36: PM
CBSE 12-science - Chemistry
Asked by dhruvrana348 | 28 Jun, 2020, 08:58: AM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 06 Jan, 2020, 15:39: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 18 Sep, 2019, 22:02: PM
CBSE 12-science - Chemistry
Asked by sanjeet.kumar | 12 Mar, 2019, 14:20: PM
CBSE 12-science - Chemistry
Asked by manpreetkaur19971993 | 10 Jan, 2019, 07:06: AM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 22 Jul, 2018, 20:27: PM