CBSE Class 12-science Answered
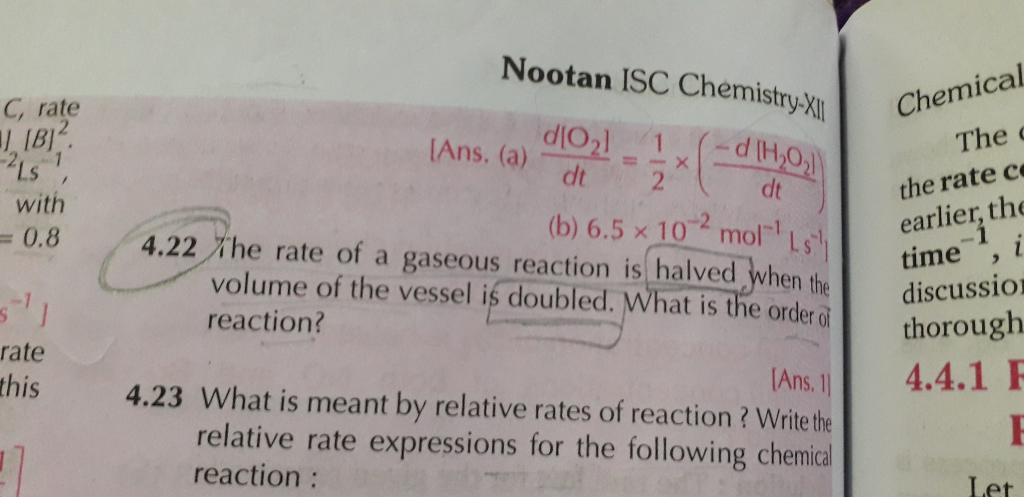
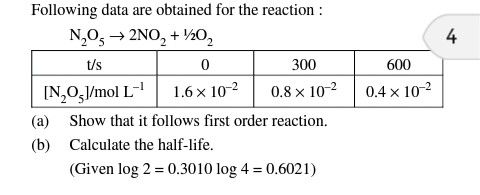
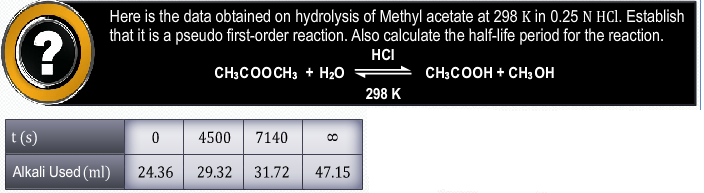
4.32 numerical plz

Asked by lovemaan5500 | 18 Sep, 2019, 22:02: PM
50% of reaction gets completed in 2 hr. that is, half life period of reaction is 2 hr.
Next half reaction gets completed in 2hr that is (25% half of reamaining 50%) half life period is 2 hr.
So the half life period of reaction is independent of concentration. Hence it is first order reaction.
Also, we can solve this by using equations for first order reaction,
We have,
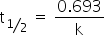
We know,
t1/2 = 2 hr
 k = 0.3465 hr−1
k = 0.3465 hr−1 By using expression for first order reaction,
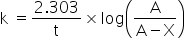
t = 4 hr
A = 100
(A-X) = 100- 75
= 25
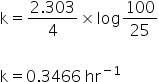
By using expressions for first order reaction, we get same value of rate constant.
Hence it is confirmed that, it is a first order reaction.
Answered by Varsha | 19 Sep, 2019, 13:12: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by BABUYVU | 02 Jun, 2024, 09:30: AM
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by arunhys123 | 04 Jul, 2020, 19:36: PM
CBSE 12-science - Chemistry
Asked by dhruvrana348 | 28 Jun, 2020, 08:58: AM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 06 Jan, 2020, 15:39: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 18 Sep, 2019, 22:02: PM
CBSE 12-science - Chemistry
Asked by sanjeet.kumar | 12 Mar, 2019, 14:20: PM
CBSE 12-science - Chemistry
Asked by manpreetkaur19971993 | 10 Jan, 2019, 07:06: AM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 22 Jul, 2018, 20:27: PM