CBSE Class 12-science - Order and Molecularity of Reaction Videos
Order and Molecularity of Reaction
This video explains effect of concentration of reactant of reaction, rate law, Order and Molecularity of reaction, Elementary of complex reaction
More videos from this chapter
View All- relationship between molarity and morality
-
relation between k and t for first order reaction

- Definition of zero order, 1st order and 2nd order reaction
- The half life for a first order reaction is 10 mins .What percentage of reactant will be left behind after 60mins.
-
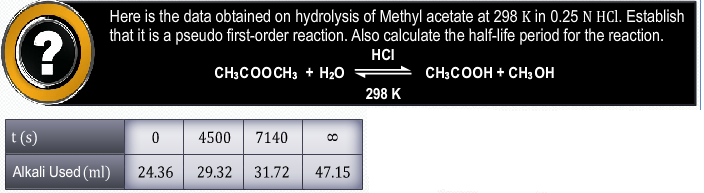
4.22 sum
-
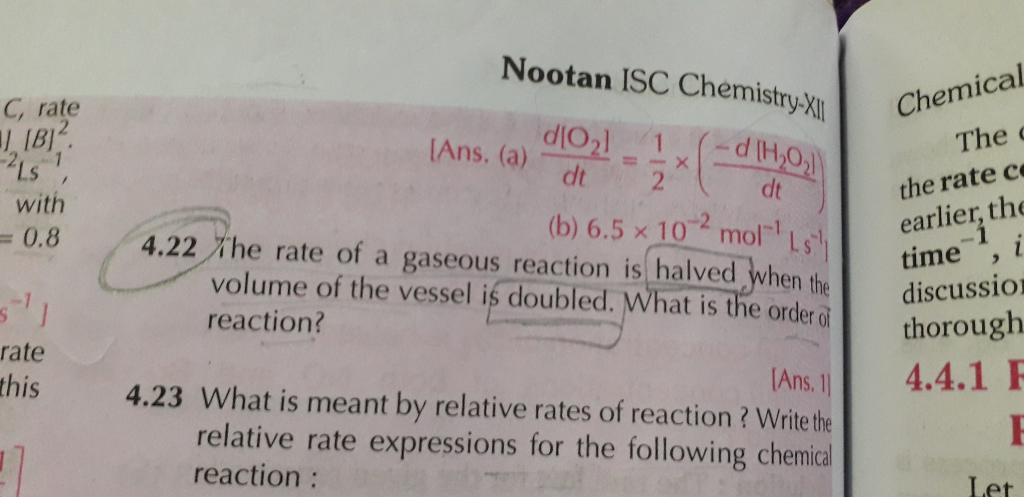
4.32 numerical plz

- Write a condition under which a bimolecular reaction is kinetically first order. Give an example of such a reaction
-
Pls answer
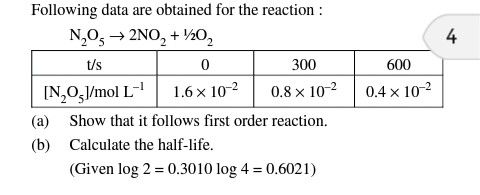
- what is the order of reaction if rate of reaction becomes 3 times and concentration of reaction becomes 9 times
-
please solve it