CBSE Class 12-science Chemistry Collision Theory
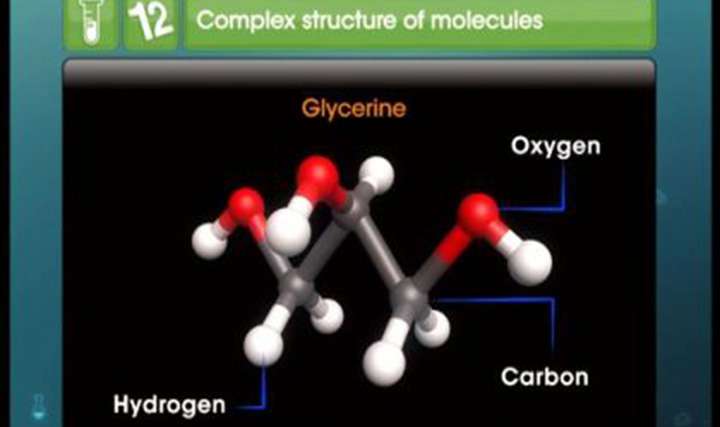
Understand the complex structure of molecules with TopperLearning’s invaluable study resources for CBSE Class 12 Science Chemistry Chemical Kinetics – Collision Theory. Our well-designed video lessons will bolster your knowledge of collision theory of chemical reactions. Through our video lessons, topic notes and textbook solutions, learn concepts like collision frequency and activation energy.
In addition, learn what the Arrhenius equation tells you about the collision behaviour of complex molecules with our CBSE Class 12 Science learning resources. For exam support, self-assessment resources like sample papers, previous years’ papers and Chemistry practice tests are easily accessible online on our learning portal 24/7.
- Collision theory of chemical reactions is based on which theory?
- Define Collision frequency Z.
- What is the main drawback of the Collision theory?
- Write down the modified form of Arrhenius equation on the basis of collision theory.
-
Hydrogen gas and iodine vapour combine to form hydrogen iodide gas, as shown in the equation H2 + I2 → 2HI. Using representations shown below draw a diagram to show an orientation for the reactant molecules that can produce an effective collision capable of producing two hydrogen iodide molecules.
- What are the two conditions that are necessary for effective collisions?
- State the main postulates of the collision theory.
- The value of the rate constant for the decomposition of nitrogen pentoxide is 3.4 ×10-5 at 250C and 4.87 × 10-3 at 65oC. Calculate the activation energy for the reaction. (R = 8.314 JK-1 mol-1). N2O5 → N2O4 + 1/2 O2
- The rate of a reaction becomes four times when the temperature changes from 293 K to 313 K. Calculate the energy change of the reaction assuming that it does not change with temperature.
- Calculate the activation energy of a hydrogen carbon whose decomposition is given as follows: K= (4.5 x 1011 s-1) e-28000K/T