CBSE Class 12-science Answered
Calculate the activation energy of a hydrogen carbon whose decomposition is given as follows: K= (4.5 x 1011 s-1) e-28000K/T
Asked by Topperlearning User | 28 Mar, 2014, 01:50: PM
According to Arrhenius equation k = A.e –Ea/RT
Comparing the given value of k = (4.5 x 1011 s-1) e-28000 K/T, we get,
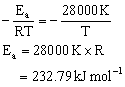
Answered by | 28 Mar, 2014, 03:50: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:13: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 01:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:15: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 01:39: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:13: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:13: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 01:50: PM