CBSE Class 12-science Chemistry Rate of Chemical Reaction
Absorb concepts such as rate reaction, average rate, instantaneous rate etc. through TopperLearning’s CBSE Class 12 Science Chemistry Chemical Kinetics – Rate of Chemical Reaction study materials. You can watch our concept videos and listen to our experts explain the concept of rate of chemical reaction.
Learn the steps to calculate average rate and instantaneous rate with our CBSE Class 12 Science Chemistry topic notes. Whether you wish to practise MCQs or skim through the concepts in the NCERT textbook solutions, we have got you covered. In addition, to give you maximum support in your Chemistry exam preparation, our learning portal gives you access to downloadable sample papers and previous years’ papers.
- effect of cone temperature of the rate of b/w sodium thiosulphur and hydrochloric acid.
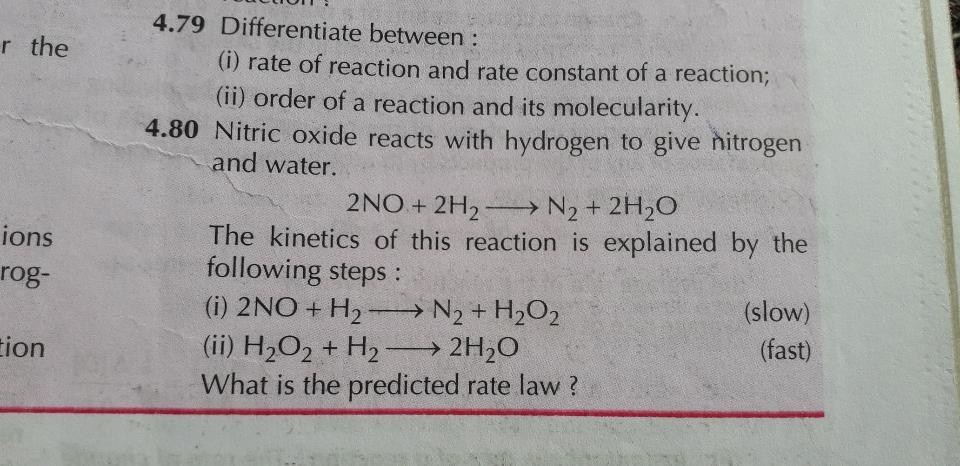
- distinction between rate of reaction and rate constant
- Of 50% of reaction gets completed in 16 minutes what fraction of reaction would occur in 32 minutes
-
Please solve question 25

- Write integrated rate expression for the first order reaction. Also, findHalf life for this expression.
-
the given questions

- rate equation
-
pls solve
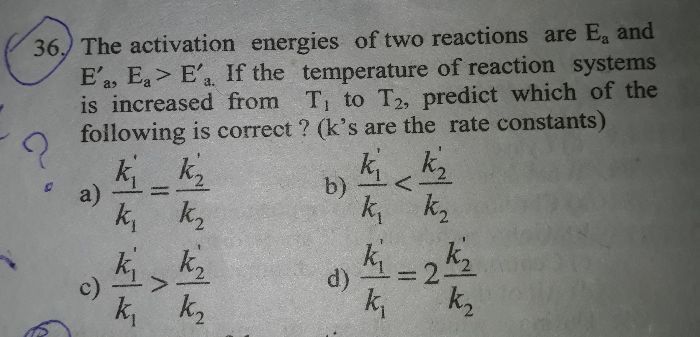
- In the reaction A + 2B 2C + D. If the concentration of A is increased four times and B is decreased to half of its initial concentration then the rate becomes
-
4.80 ques