CBSE Class 12-science Chemistry Temperature Dependence of Reaction
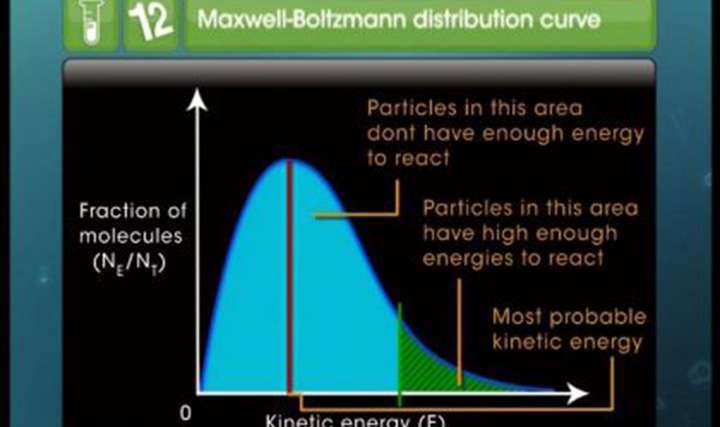
Watch our interesting concept videos to enjoy learning CBSE Class 12 Science Chemistry Chemical Kinetics – Temperature Dependence of Reaction concepts. Strengthen your Chemistry basics by learning the Maxwell–Boltzmann distributions through our video lessons and other learning materials.
Relearn the effect of a catalyst on the rate of reaction by using our CBSE Class 12 Science Chemistry topic notes and textbook solutions at the time of revision. Also, find out the effect of temperature on the rate of reaction. For further learning, attempt questions from our practice question papers. To keep a check on your conceptual clarity, take our Chemistry practice tests as many times as required.
- the half life span of a radioactive element is 10 years .in no. will 10 gram of that substances remain 1•25 gram
- what is the formula for effect of temperature on rate of reaction?
- Define activation energy.
- What is the effect of a catalyst on the equilibrium constant of a reaction?
- What is temperature coefficient? Explain
- Define transition state or activation complex.
- The activation energy for a reaction is zero. Calculate the value of its rate constant at 300 K, if k = 1.6 x 106 s-1 at 280 K? (R = 8.31 JK-1 Mol-1)
-
Draw a graph to show the various energy changes that take place in the following reaction:
H2 (g) + I2 (g)
 2HI (g)
2HI (g)
- Explain why the activation energy of a reaction can never be zero.
-
The rate constant for the first order decomposition of certain reaction is described by the equation log k (sec-1) =
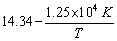 Calculate the activation energy for this reaction.
Calculate the activation energy for this reaction.