CBSE Class 12-science Answered
The value of the rate constant for the decomposition of nitrogen pentoxide is 3.4 ×10-5 at 250C and 4.87 × 10-3 at 65oC. Calculate the activation energy for the reaction. (R = 8.314 JK-1 mol-1).
N2O5 → N2O4 + 1/2 O2
Asked by Topperlearning User | 22 Jun, 2016, 09:13: AM
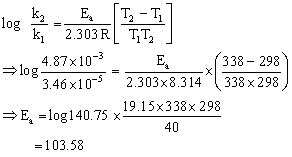
Answered by | 22 Jun, 2016, 11:13: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:13: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 01:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:15: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 01:39: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:13: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:13: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 01:50: PM