CBSE Class 11-science Chemistry Measurement of dU and dH
-
50 j of heat is supplied to a system calculate the work done if the internal energy of the system increases by 80 j

-
n
-
pl experts ans as soon as possible
- A system absorb 20 kJ heat and also does 10 kJ of work. The net internal energy of the system is :- (1) increases by 10 kJ (2) decreases by 10 kJ (3) increases by 30 kJ (4) decreases by 30 kJ
- a system absorbs 500J of heat and does work of 50J on its surroundings. calculate the change in internal energy
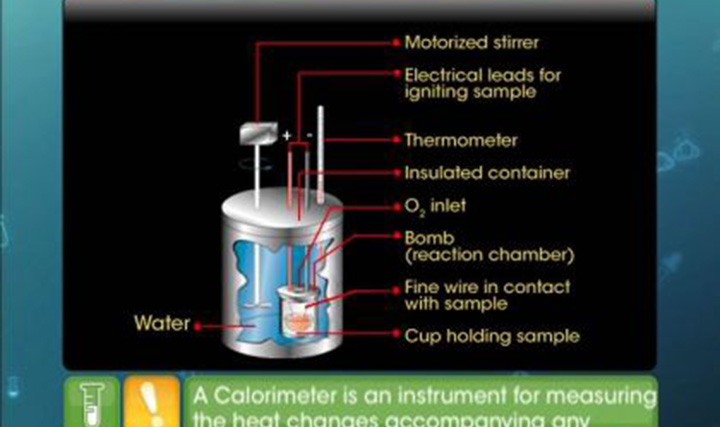
- What is the use of a bomb calorimeter?
- Define standard enthalpy of fusion.
- How can we find out the atomicity of the gases from their molar heat capacities?
- On burning two moles of hydrogen with one mole of oxygen form two moles of liquid water and liberate 13,663 calories of heat. Write the thermochemical equation for this reaction.
-
Calculate the amount of heat required for making 1kg of calcium carbide according to the following reaction.
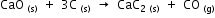 The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.
The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.