CBSE Class 11-science Answered
Define standard enthalpy of fusion.
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
The enthalpy change that accompanies melting of one mole of a solid substance in standard state is called standard enthalpy of fusion.
Answered by | 15 Jun, 2016, 19:22: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kjay0981 | 13 Dec, 2020, 15:45: PM
CBSE 11-science - Chemistry
Asked by jain.pradeep | 14 Apr, 2019, 00:33: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 00:31: AM
CBSE 11-science - Chemistry
Asked by gganga | 13 Apr, 2018, 18:34: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 13:38: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:12: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:32: PM

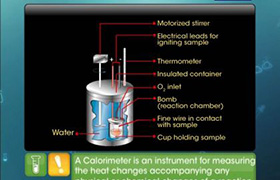
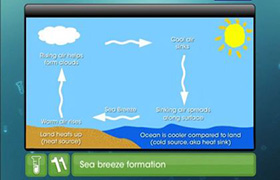

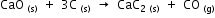 The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.
The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.