CBSE Class 11-science Answered
a system absorbs 500J of heat and does work of 50J on its surroundings. calculate the change in internal energy
Asked by gganga | 13 Apr, 2018, 18:34: PM
Change in energy given by:
dQ = du + dw
dQ = 500 J
dw = - 100 J
du = dQ - dw
= 500 - ( -100 )
= 600 J
Answered by Ramandeep | 14 Apr, 2018, 18:45: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kjay0981 | 13 Dec, 2020, 15:45: PM
CBSE 11-science - Chemistry
Asked by jain.pradeep | 14 Apr, 2019, 00:33: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 00:31: AM
CBSE 11-science - Chemistry
Asked by gganga | 13 Apr, 2018, 18:34: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 13:38: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:12: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:32: PM



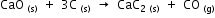 The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.
The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.