CBSE Class 11-science Answered
50 j of heat is supplied to a system calculate the work done if the internal energy of the system increases by 80 j

Asked by kjay0981 | 13 Dec, 2020, 15:45: PM
According to first law of thermodynamics,
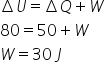
W is positive which means 30 J work done on system
Answered by Ravi | 16 Dec, 2020, 11:47: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by kjay0981 | 13 Dec, 2020, 15:45: PM
CBSE 11-science - Chemistry
Asked by jain.pradeep | 14 Apr, 2019, 00:33: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 00:31: AM
CBSE 11-science - Chemistry
Asked by gganga | 13 Apr, 2018, 18:34: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 13:38: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:12: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:32: PM

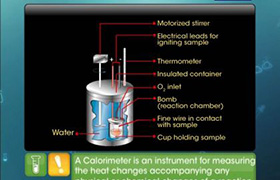

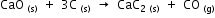 The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.
The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.