CBSE Class 11-science Answered
A system absorb 20 kJ heat and also does 10 kJ of work. The net internal energy of the system is :-
(1)
increases by 10 kJ
(2)
decreases by 10 kJ
(3)
increases by 30 kJ
(4)
decreases by 30 kJ
Asked by Atulcaald | 25 May, 2018, 00:31: AM
Option (1) is correct.
Internal energy increases by 10 kJ.
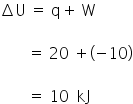
Answered by Varsha | 25 May, 2018, 11:51: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by kjay0981 | 13 Dec, 2020, 15:45: PM
CBSE 11-science - Chemistry
Asked by jain.pradeep | 14 Apr, 2019, 00:33: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 00:31: AM
CBSE 11-science - Chemistry
Asked by gganga | 13 Apr, 2018, 18:34: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 13:38: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:12: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:32: PM



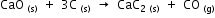 The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.
The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.