CBSE Class 11-science Answered
On burning two moles of hydrogen with one mole of oxygen form two moles of liquid water and liberate 13,663 calories of heat. Write the thermochemical equation for this reaction.
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
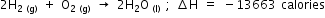
Answered by | 15 Jun, 2016, 19:22: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kjay0981 | 13 Dec, 2020, 15:45: PM
CBSE 11-science - Chemistry
Asked by jain.pradeep | 14 Apr, 2019, 00:33: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 00:31: AM
CBSE 11-science - Chemistry
Asked by gganga | 13 Apr, 2018, 18:34: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 13:38: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:12: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 14:32: PM



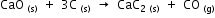 The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.
The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.