CBSE Class 11-science Chemistry Entropy and Gibbs Energy
-
what is the equilibrium constant k
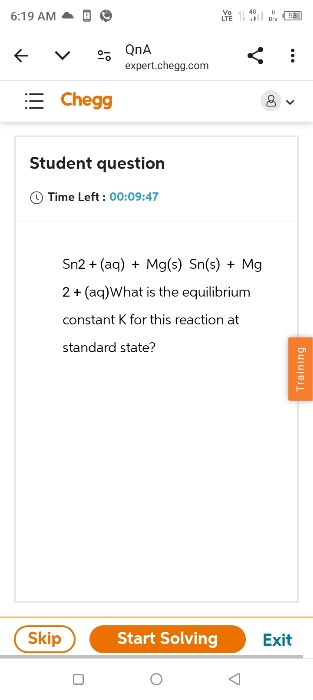
- the total entropy change for system it's sourunding increase if process is
- write a relation between delta G and Q and define the meaning of each term and answer the following a) why a reaction proceeds forward when Q< K and no net reaction occurs when Q = K b) explain the effect of increase in pressure in terms of reaction quotient Q for the reaction CO ? CH4(g) + H2O(g) (ii) for the reaction, N2(g) +3H2(g)? 2NH(g) at 400K , Kp = 41, find the value Kp for the reaction 2N2(g) +6H2(g) ? 4NH3(g)
- Give the relationship between Gibbs energy change and equilibrium constant?
-
What is the relation between
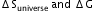 ?
?
- At what temperature the entropy of a perfectly crystalline substance is zero?
-
For a reaction,
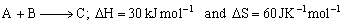 Determine the temperature above which reaction will be spontaneous.
Determine the temperature above which reaction will be spontaneous.
- Calculate the entropy of vaporization of water if its enthalpy of vaporization is 186.5 kJmol-1?
-
For a water gas reaction at 1000°C the standard Gibb’s energy change is -8.1 kJ mol-1. Calculate the value of equilibrium constant?
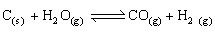
-
Calculate the standard Gibbs energy change for the following reaction:
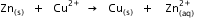 Given that
Given that 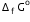 for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.
for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.




