CBSE Class 11-science - Entropy and Gibbs Energy Videos
Thermodynamics
This video explains the relation between entropy and spontaneity, Gibbs energy and spontaneity, Gibbs energy change and equilibrium.
More videos from this chapter
View All-
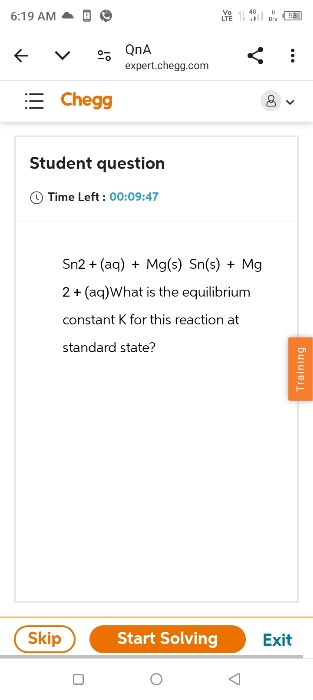
what is the equilibrium constant k
- the total entropy change for system it's sourunding increase if process is
- write a relation between delta G and Q and define the meaning of each term and answer the following a) why a reaction proceeds forward when Q< K and no net reaction occurs when Q = K b) explain the effect of increase in pressure in terms of reaction quotient Q for the reaction CO ? CH4(g) + H2O(g) (ii) for the reaction, N2(g) +3H2(g)? 2NH(g) at 400K , Kp = 41, find the value Kp for the reaction 2N2(g) +6H2(g) ? 4NH3(g)
- Give the relationship between Gibbs energy change and equilibrium constant?
-
What is the relation between
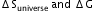 ?
?
- At what temperature the entropy of a perfectly crystalline substance is zero?
-
For a reaction,
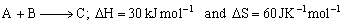 Determine the temperature above which reaction will be spontaneous.
Determine the temperature above which reaction will be spontaneous.
- Calculate the entropy of vaporization of water if its enthalpy of vaporization is 186.5 kJmol-1?
-
For a water gas reaction at 1000°C the standard Gibb’s energy change is -8.1 kJ mol-1. Calculate the value of equilibrium constant?
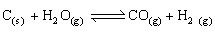
-
Calculate the standard Gibbs energy change for the following reaction:
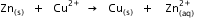 Given that
Given that 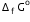 for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.
for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.