CBSE Class 11-science - Hess's Law Videos
Thermodynamics
This video explains the meaning of enthalpy, enthalpy of formation and enthalpy of combustion.
- state hess's law. how does it follow the first law of thermodynamics.
- Give an example of application of Hess' Law.
- Explain with example Enthalpy of Solution.
- Compare lattice Enthalpy and Ionization Enthalpy.
- The enthalpy of combustion for H2, C (graphite) and CH4 are -285.8, -393.5, and -890.4 kJ/mol respectively. Calculate the standard enthalpy of formation ∆Hf for CH4.
- Enthalpy of combustion of carbon graphite and carbon diamond is -293.5(kJ/mol) and - 670(kJ/mol) respectively. Calculate conversion of graphite into diamond.
- Define bond dissociation energy and mean bond dissociation energy.
- State "The Hess's law of constant summation".
-
Show calculation of
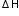 from: Enthalpy changes of formation and bond enthalpies.
from: Enthalpy changes of formation and bond enthalpies.
-
Calculate the
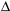 H of the following reaction, which describes the production of coal gas from carbon, given the steps below.
H2O (g)
+
C (s)
H of the following reaction, which describes the production of coal gas from carbon, given the steps below.
H2O (g)
+
C (s)
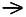 CO (g)
+
H2 (g)
Given Steps
H2 (g)
+
1/2 O2 (g)
CO (g)
+
H2 (g)
Given Steps
H2 (g)
+
1/2 O2 (g)
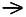 H2O (g)
H2O (g)
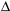 H
=
- 242.0 kJ
2CO (g)
H
=
- 242.0 kJ
2CO (g)
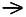 2 C (s)
+
O2 (g)
2 C (s)
+
O2 (g)
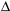 H
=
+ 221.0 kJ
H
=
+ 221.0 kJ

