CBSE Class 11-science Answered
write a relation between delta G and Q and define the meaning of each term and answer the following
a) why a reaction proceeds forward when Q< K and no net reaction occurs when Q = K
b) explain the effect of increase in pressure in terms of reaction quotient Q for the reaction CO ? CH4(g) + H2O(g)
(ii) for the reaction,
N2(g) +3H2(g)? 2NH(g)
at 400K , Kp = 41, find the value Kp for the reaction
2N2(g) +6H2(g) ? 4NH3(g)
Asked by gganga | 10 Apr, 2018, 18:02: PM

Answered by Varsha | 11 Apr, 2018, 13:10: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by anithaanu629940 | 23 May, 2024, 08:33: AM
CBSE 11-science - Chemistry
Asked by mankdubey670 | 06 Jun, 2022, 13:27: PM
CBSE 11-science - Chemistry
Asked by gganga | 10 Apr, 2018, 18:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 16:44: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:46: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 16:51: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 16:53: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 17:00: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 17:46: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Sep, 2014, 15:28: PM




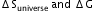 ?
?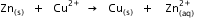 Given that
Given that 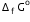 for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.
for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.