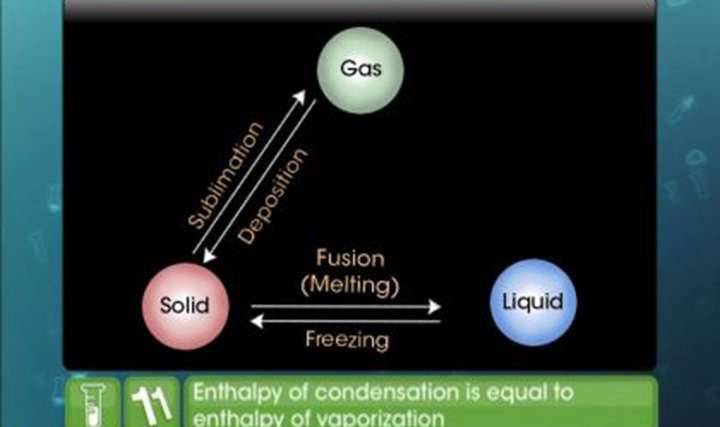
CBSE Class 11-science Chemistry Enthalpy Change
- Que: Differentiate between enthalpy and entropy??
- PMM2 kind is impossible according to
- a steel fiber is in a oxygen filled container which is closed with a frictionless piston. the iron in the steel fiber reacts with oxygen to form Fe2O3 , the heat generated by the reaction is removed during the process to maintain the temperature constant which is 25 degrees centigrade. for the reaction of 2 moles of iron 831.08KJ of heat is removed. calculate the heat , work and internal energy of change of the system
-
How to solve this Question?
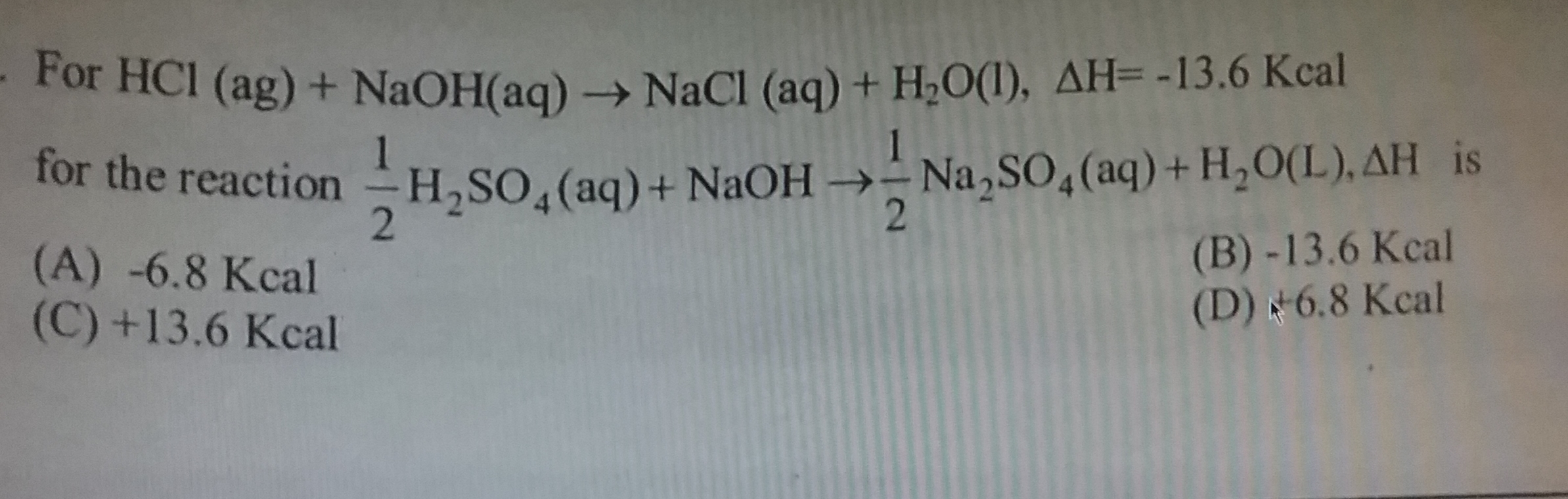
- When 100ml of strong acid mixed with 100ml of strong base, change in temperature is 5°c. Determine the change in temperature when 10ml of strong acid mixed with strong base.
-
Please answer
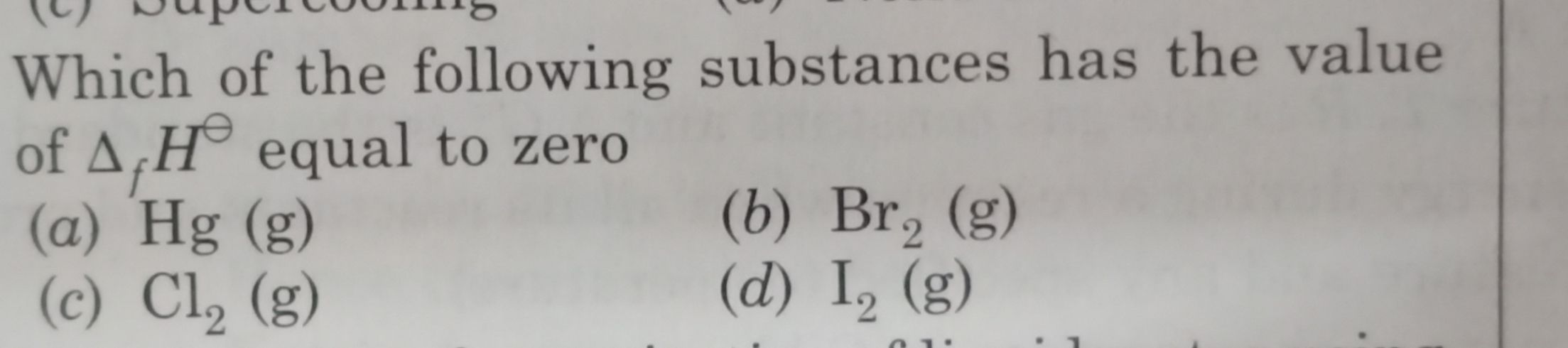
- How is isothermal irreversible expansion cyclic and how is delta H=0 for the process?
-
The transition Sn(s, gray) ⇌ Sn(s, white) is in equilibrium at 18°C and 1 atm pressure. If ΔS = 8.811K mol for the transition at 18°C and if the densities are 5.75 g/cm3 for gray tin and 7.28 g/cm3 for white tin, calculate the transition temperature under 100 atm pressure

-
6.63 sum
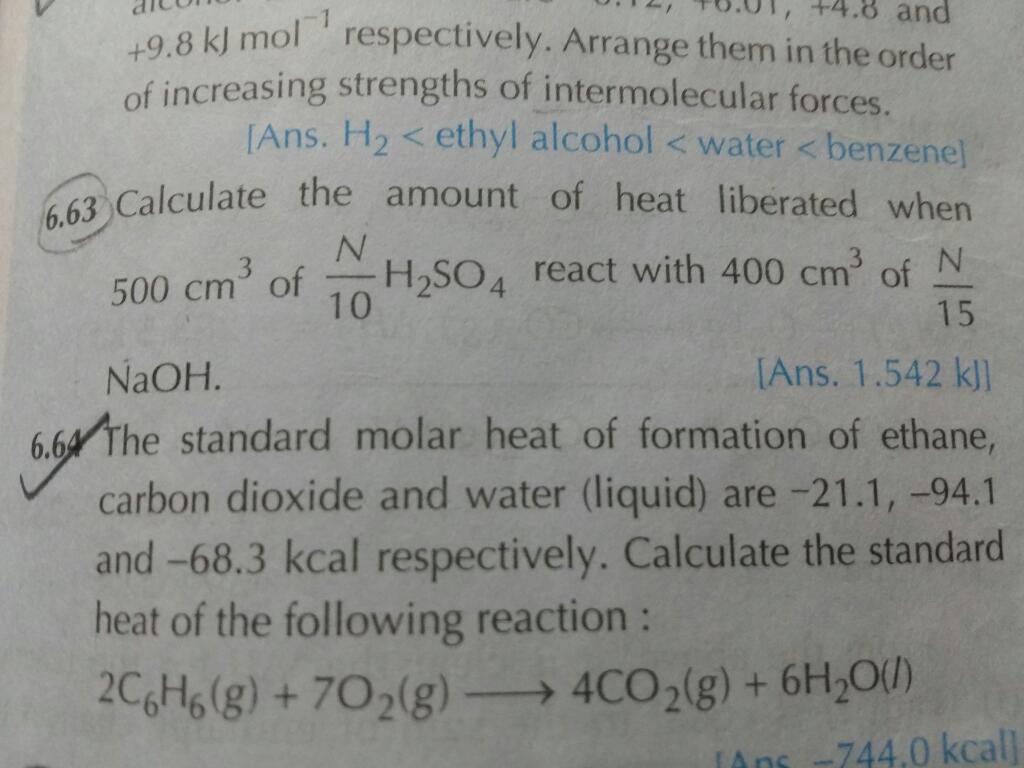
- One mole of anhydrous MgCl2 dissolves in water and librates 25 cal/mol of heat. ∆Hhydration of MgCl2 = –30 cal/mol. Heat of dissolution of MgCl2.7H2O(s) is :- (1) +5 cal/mol (2) –5 cal/mol (3) 55 cal/mol (4) –55 cal/mol