States of Matter
States of Matter PDF Notes, Important Questions and Synopsis
SYNOPSIS
- Boyle’s law: At constant temperature, pressure is inversely proportional to volume for a definite amount of ideal gas.
- Charles’ law: At constant pressure, volume is directly proportional to temperature for a definite amount of ideal gas.
- Gay-Lussac’s law: For a given mass and constant volume, pressure is directly proportional to temperature.
- Avogadro’s law: At the same pressure and temperature, equal volumes of ideal gases consist of equal number of moles or molecules.
- Dalton’s law of partial pressure: If two or more non-reacting gases are kept in a container, the total pressure exerted by the gases in the container is the sum of their partial pressures.
- Graham’s law of effusion and diffusion: The rate of effusion or diffusion of a gas is inversely proportional to the square root of molecular mass or density when compared at the same temperature and pressure.
- Kinetic Theory of Gases:
- A molecule is the smallest particle of the gas.
- Molecules do not settle under the influence of gravity.
- Molecules move in a straight line until or unless they collide with each other or with the wall of the container.
- The volume of gas molecules is very small, so it can be neglected in comparison to the volume of the container.
- Collisions of gas molecules with each other or collisions between gas molecules and the wall of the container are perfectly elastic.
- Kinetic energy of gas molecules is directly proportional to temperature.
- There are neither attractive nor repulsive forces present between gas molecules.
- Root mean square speed: It is calculated by first taking squares of individual velocities, taking their mean and then the square root of the mean.
- Average speed: It is calculated by taking the arithmetic mean of the speeds of different molecules of the gas.
-
Most probable speed: It is the speed possessed by maximum number of gas molecules at a given temperature.
-
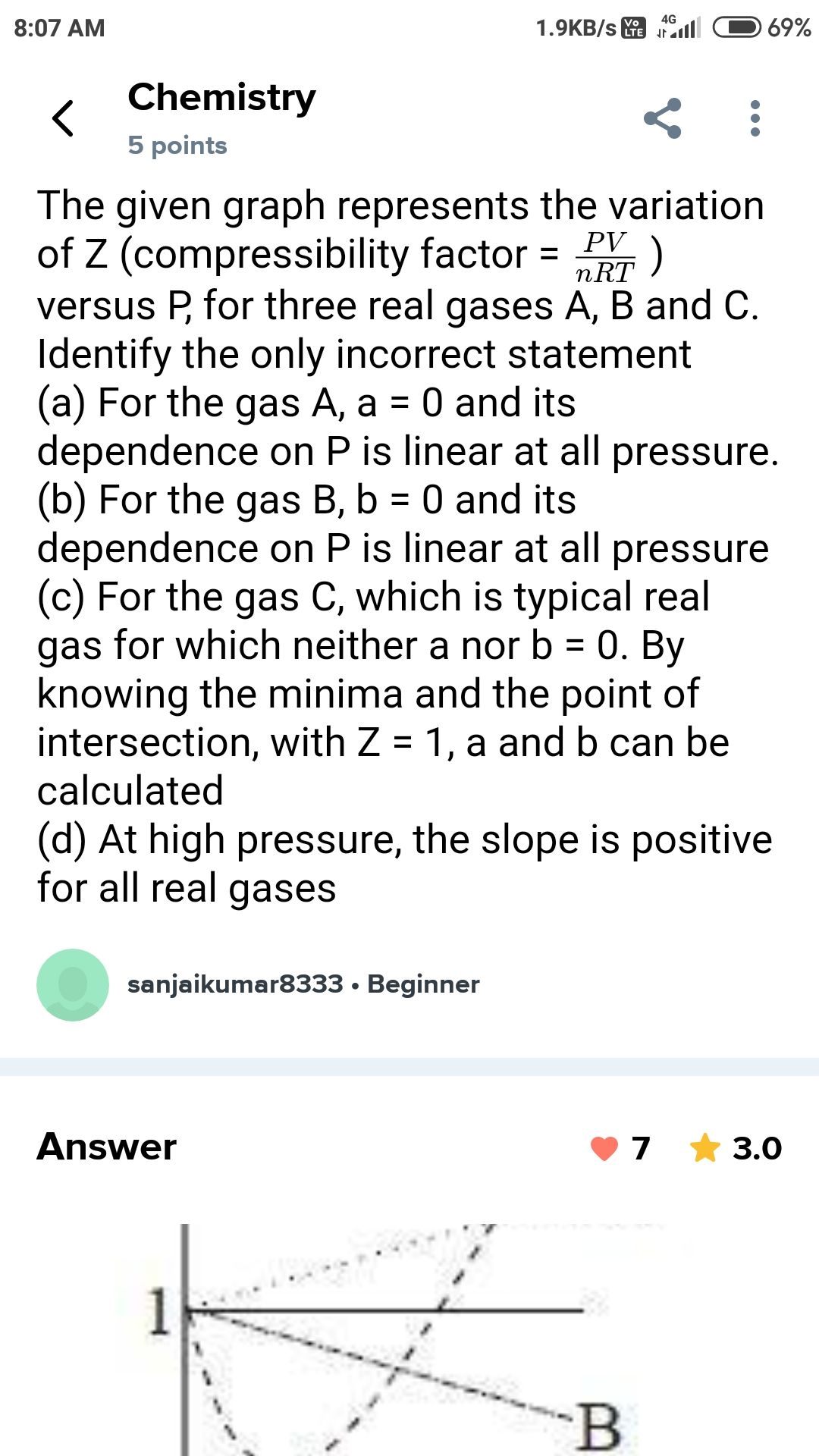
Compressibility factor (Z): It is the ratio of observed volume of a gas to the calculated volume under given conditions of temperature and pressure of the gas.
-
Critical temperature: The temperature above which a gas cannot be liquefied no matter how much pressure is applied.
-
Critical pressure: Pressure exerted by gas at critical temperature.
-
Critical volume: Volume attained by gas at critical temperature and pressure.
-
Vapour pressure: It is the pressure exerted by a vapour when vapour is in equilibrium with the liquid or solid form or both.
-
Boiling point: The temperature at which the vapour pressure of a liquid becomes equal to the atmospheric pressure.
-
Freezing point: The temperature at which the vapour pressure of a liquid becomes equal to the vapour pressure of the solid.
-
Surface tension: It is the tendency of a fluid surface to occupy the smallest possible area.
-
Viscosity: It is the property of liquids which determines their resistance to flow.
Related Chapters
- Some Basic Concepts in Chemistry
- Atomic Structure
- Chemical Bonding and Molecular Structure
- Chemical Thermodynamics
- Solid State
- Solutions
- Equilibrium
- Redox Reactions and Electrochemistry
- Chemical Kinetics
- Surface Chemistry
- Classification of Elements and Periodicity in Properties
- General Principles and Processes of Isolation of Metals
- Hydrogen
- s-Block Element (Alkali and Alkaline Earth Metals)
- p-Block Elements
- d - and f - Block Elements
- Co-ordination Compounds
- Environmental Chemistry
- Purification and Characterisation of Organic Compounds
- Some Basic Principles of Organic Chemistry
- Hydrocarbons
- Organic Compounds Containing Halogens
- Organic Compounds Containing Oxygen
- Organic Compounds Containing Nitrogen
- Polymers
- Biomolecules
- Chemistry in Everyday Life
- Principles Related to Practical Chemistry